0606
Topography of the acoustic radiation as revealed by ex-vivo fiber dissections and in-vivo diffusion-based tractography1CIMeC Center for Mind/Brain Sciences, Trento University, Trento, Italy, 2Division of Neurosurgery, Bambino Gesù Hospital, Rome, Italy, 3Structural and Functional Connectivity Lab, Div. of Neurosurgery, “S.Chiara Hospital, Trento APSS, Italy
Synopsis
The acoustic radiation (AR) is a compact bundle of fibers conveying auditory information from the thalamus to the cortex. Topographical knowledge of this bundle is scarce and its diffusion-based tractographic reconstruction remains hardly achievable, especially for commonly available MRI acquisition protocols. In this scenario, validation of tractography results is particularly important. In this study we used blunt dissection to precisely characterize AR topography and relationships with adjacent bundles. Being aware of the anatomical characteristics of the tract provides us with the underlying ground truth on which methodological decisions, aimed at overcoming the limits of the tractographic reconstruction, can be made.
Purpose
The acoustic radiation (AR) constitutes a primary sensory projection pathway, transferring auditory information from the medial geniculate nucleus (MGN) of the thalamus to the auditory cortex [transverse temporal gyrus of Heschl (HG)]. While the connectivity pattern of these fibers has been described in some detail in cytoarchitectonic and myeloarchitectonic studies1,2 in non-human primates, very little is known about its topography in humans. Diffusion tractography techniques allow the investigation of the white matter (WM) architecture of the human brain in vivo. However the diffusion-based tractographic reconstruction of the AR remains extremely challenging3,4. In this scenario, where limited topographical knowledge is available, validation of tractography results is of great importance. The aims of this study are 1) to characterize the topography of the AR relative to other major WM bundles in the human brain, and 2) to compare the results of ex-vivo dissections with the diffusion-based 3-D reconstructions.Methods
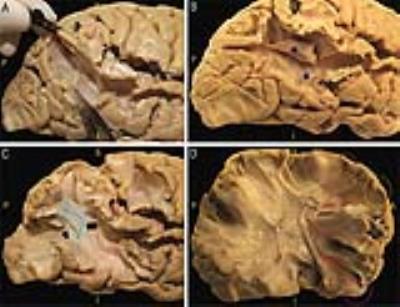
Acoustic radiation fiber dissection: Three human cerebral hemispheres (two right) were prepared according to a modified Klinger’s technique5. AR was approached posteriorly starting a layer-by-layer dissection from the posterior third of the superior temporal sulcus (STS) and its elongation in the inferior parietal lobe (IPL)6. U-fibers connecting the supramarginal gyrus (SMG) and the angular gyrus (AG) with the cortices of the posterior two-thirds of the STS were removed (Figure 1A). The stem of the vertical portion of the superior longitudinal fasciculus (SLF) was highlighted and cut (Figure 1B), demonstrating the temporal portion of the arcuate fascicle (AF) (Figure 1C). The deepest portion and the most ventral fibers of the AF, connecting the frontal lobe to the posterior third of STG (Figure 1D), were removed to highlight the thalamic radiation and the AR fibers projecting to HG.
Acoustic radiation tractography: MRI data of three subjects provided by the Human Connectome Project were analyzed using MrTrix7. The HARDI multi-shell dataset is composed of four shells (1000-3000-5000-10000 s/mm2) for a total of 552 directions at an isotropic spatial resolution of 1.25 mm8. A multi-shell multi-tissue constrained spherical deconvolution algorithm9 was fit to the data and anatomically-constrained10 probabilistic tractography was performed ( 0.75 mm step-size, 45° angle threshold, 1000 seeds/voxel). The thalamus was segmented in FSL from each subject’s anatomical T1 while HG was manually defined. Only fibers passing through these regions were retained.
Results
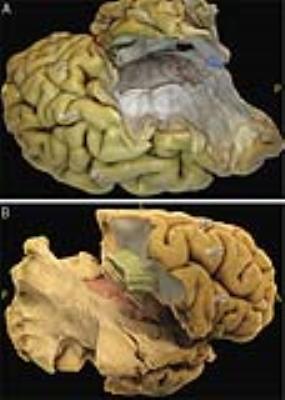
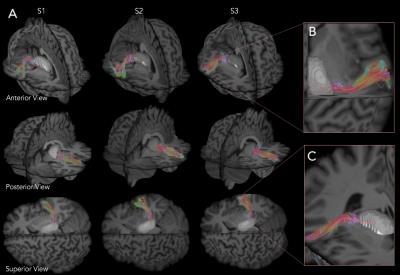
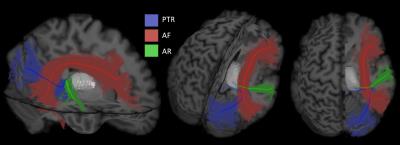
After removal of the ventral fibers of AF we found: medially, the compact layer of vertical fibers projecting from and to the thalamus; antero-laterally, a transverse layer of AR fibers starting from the postero-lateral portion of the thalamus (MGN) (Figure 2). Dissection demonstrated a typical course of the AR. In the first third of their course these fibers are compact and project laterally. Then these run in an antero-lateral direction funning to the whole cortex of HG. On the coronal plane we appreciated a straight medio-lateral course from the origin to the termination site. The fibers of the proximal third are intermingled to the vertical fibers of the thalamic radiation (Figure 2B). The middle third of AR run anteriorly to the AF stem, below the ventral portion of the fronto-parietal course of AF and SLF III. Ultra-high b-value and constrained spherical deconvolution tractography allowed the reconstruction of the AR profile in all subjects (Figure 3A). In accordance to the blunt dissections, the streamlines originate as a compact bundle at the level of the MGN and move in a lateral and anterior direction, fanning out in the grey matter of HG (Figure 3B). Tractography reconstructions confirmed the origin and termination of this bundle is on the same plane but highlighted a typical s-shape course, due to AR fibers arching around the postero-superior portion of the circular sulcus of the insula (Figure 3C). The AR’s relationship with bordering bundles is also confirmed by tractography reconstructions (Figure 4).Discussion and Conclusion
To our knowledge this is the first study revealing the topography of the AR in the human brain combining ex-vivo dissections and diffusion tractography. Both methods showed the AR unique feature of a full transversal course from the midline to the lateral convexity of the posterior portion of the superior temporal lobe. The challenges in tractographic reconstruction are strictly related to this complex anatomical profile and to the critical texture of multi-directional pathways. Dissections demonstrated, in fact, that AR is located in a region with a high density of crossing fibers: close to compact layers of vertical thalamo-cortical fibers, and at the termination site of different and multi-layers associative pathways. Solving the fiber-crossing in these regions and defining the tract terminations represents an open challenge for tractography algorithms.Acknowledgements
No acknowledgement found.References
1. Hackett T. A., Stepniewska I., & Kaas J. H. Thalamocortical connections of the parabelt auditory cortex in macaque monkeys. The Journal of Comparative Neurology. 1998b; 400(2), 271-86.
2. Mesulam M. M., & Pandya, D. N. The projections of the medial geniculate complex within the Sylvian Fissure of the Rhesus monkey. Brain Research. 1973; 60, 315-333.
3. Berman, J. I., Lanza M. R., Blaskey L. et al. High angular resolution diffusion imaging probabilistic tractography of the auditory radiation. American Journal of Neuroradiology. 2013a; 34(8), 1573-1578.
4. Behrens T. E. J., Berg H. J., Jbabdi S., et al. Probabilistic diffusion tractography with multiple fiber orientations: What can we gain? 2007; 34(1), 144-155.
5. Sarubbo S. et al. The course and the anatomo-functional relationships of the optic radiation: a combined study with ‘opst mortem’ dissections and ‘in vivo’ direct electrical mapping. Journal of Anatomy. 2015; 226(1), 47-59.
6. Sarubbo S., De Benedictis A., Merler S. et al. Structural and functional integration between dorsal and ventral language streams as revealed by blunt dissection and direct electrical stimulation. Human Brain Mapping. 2016; 37(11):3858-3872.
7. Tournier J. D, Calamante F., and Connelly A. MRtrix: Diffusion tractography in crossing fiber regions. Int. J. Imaging Syst. Technol. 2012; 22(1), 53–66.
8. Fan Q., Witzel T., Nummenmaa A., et al. MGH-USH Human Connectome Project datasets with ultra-high b-value diffusion MRI
9. Jeurissen, B., Tournier, J. D., Dhollander, T., et al. Multi-tissue constrained spherical deconvolution for improved analysis of multi-shell diffusion MRI data. NeuroImage. 2014; 103, 411-426
10. Smith R. E., Tournier J. D., Calamante F., & Connelly A. Anatomically-constrained tractography: Improved diffusion MRI streamlines tractography through effective use of anatomical information. Neuroimage. 2012; 62(3), 1924-1938.
Figures