0598
Predicting Functional and Histological Outcomes in Spinal Cord Injury: Comparing Double Diffusion Encoding and Diffusion Tensor Imaging in the Rat1Biophysics Graduate Program, Medical College of Wisconsin, Milwaukee, WI, United States, 2Medical Scientist Training Program, Medical College of Wisconsin, Milwaukee, WI, United States, 3Neurosurgery, Medical College of Wisconsin, Milwaukee, WI, United States, 4Clement J Zablocki Veterans Affairs Medical Center, Milwaukee, WI, United States, 5Biomedical Engineering, Marquette University, Milwaukee, WI, United States
Synopsis
Using a rat model of spinal cord injury (SCI), diffusion tensor imaging (DTI) is compared to double diffusion encoding (DDE) at the acute and chronic stages after injury. Acute DDE measurements show a strong relationship with chronic functional outcomes whereas DTI has poor prognostic sensitivity. On the other hand, during the chronic stage, DTI outperforms DDE as a marker of functional status. The differences reflect evolving pathologies that must be considered for the appropriate application and interpretation of DTI and DDE. The results also highlight the prognostic potential of DDE in acute SCI.
Purpose
Early detection of microstructural axonal damage following spinal cord injury (SCI) is an important unmet clinical challenge in predicting long-term functional recovery1,2. Diffusion-weighted imaging (DWI) has provided advances towards this goal, however its specificity for axonal damage is often confounded by edema and inflammatory processes. This work compares diffusion tensor imaging (DTI) with double diffusion encoding using an orthogonal filter-probe (DDE-OFP) encoding scheme3 to assess the ability to predict pathological and functional outcomes in a rat SCI model.Methods
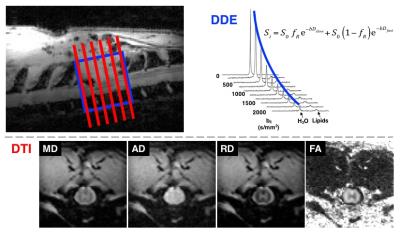
A Bruker 9.4T Biospec was
used for imaging the thoracic spine of rats in
vivo using a quadrature volume coil for transmission and 4-channel surface
coil array for reception. Rats underwent graded weight-drop injuries at the T10
vertebral level and were imaged 2 and 30 days post-injury (n=14).
DDE-OFP was implemented with two pairs of
Stejskal-Tanner gradients (δ/Δ = 12/6 ms for each) as described previously3.
Using a Point RESolved Spectroscopy (PRESS) voxel (10x10x6 mm3) with
TR/TE = 1750/42.26 ms, a b=2000 s/mm2 filter, and 9 probe b-values
ranging from 0-2000 s/mm2, acquisition time was 3 minutes. Spectra
analysis in Matlab was used to fit monoexponential and biexponential signal
equations to yield parallel diffusivity(ADC||) and diffusion
restricted fraction(fR), respectively.
DWI were acquired with a standard PGSE sequence (δ/Δ =
8.25/12.5 ms) using a 4-shot, respiratory gated EPI sequence (TR/TE = 1500/28
ms) consisting of 30 directions and 3 b-values (500, 1000, and 2000 s/mm2)
at an in-plane resolution of 0.20 mm2, 128x128 matrix, and 1.0 mm slice
thickness. Acquisition time was approximately 65 minutes. Images were corrected
for motion and eddy currents using the spinal cord toolbox4 and DTI parameter
maps calculated with FSL5(Fig. 1). Regions of interest (ROI) were
drawn manually, encompassing the whole-cord at the injury site.
The Basso,
Beattie, and Bresnahan (BBB) scoring method6 was used to evaluate
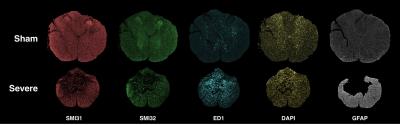
locomotor function 30 days post-injury. Perfusion fixation was
performed at 30 days post injury, and 5 μm thick tissue slices from the lesion epicenter were stained for normal
axons (SMI31), injured axons (SMI32), cellularity (DAPI), activated microglia (ED1),
and astrocytes (GFAP). 40x magnification fluorescent microscopy images were
analyzed in Matlab for total counts of positive staining cells within the
spinal cord using a threshold to remove background signal.
Linear regression analyses were used to evaluate the
relationship between diffusion measurements acquired at the acute and chronic
stages with functional and histological outcomes assessed at the chronic stage.
Results
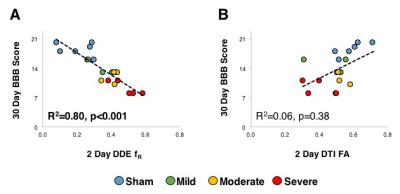
The DDE metric fR measured
at the acute timepoint (2 days) was a
significant predictor of chronic (30-day) BBB score (R2=0.80;
p<0.001), whereas neither acutely-measured FA (R2=0.06, p=0.38)
nor any of the other DTI metrics were significant predictors of functional
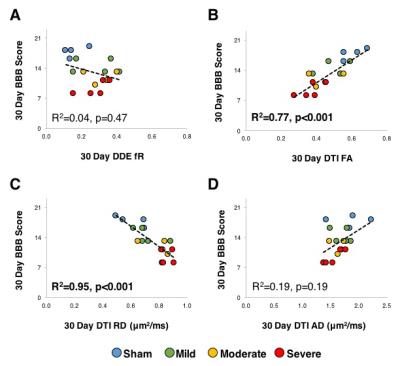
outcome. On the contrary, the DDE metric
fR measured at the chronic
timepoint was not significantly associated with chronic BBB score (R2=0.04,
p=0.47), whereas FA had a significant relationship with chronic BBB score (R2=0.77,
p<0.001). The FA changes were primarily
related to changes in radial diffusivity, since RD was significantly associated
with BBB score (R2=0.95, p<0.001) whereas AD was not (R2=0.19,
p=0.19).
As expected, histological
markers of injury showed strong associations with functional status measured as
the same chronic timepoint1,2,7.
Axonal integrity assessed with SMI31 (R2=0.35, p=0.04) and
SMI32 (R2=0.43, p=0.02) were significantly associated with BBB score
as was microglia activation assessed with ED1 (R2=64, p=0.001). Neither astrocyte proliferation assessed with
GFAP (R2=0.11, p=0.27) nor a general marker of cellularity measured
with DAPI (R2<0.01,p=0.86) were associated with BBB score. Many of the histological markers were
strongly associated with one another.
The chronically measured DTI metrics also had strong associations with
histological markers measured at the same timepoint. RD was most strongly associated with the
microglial marker ED1 (R2=0.55, p=0.002), but was also significantly
associated with axonal markers of SMI31 (R2=0.36, p=0.02) and SMI32 (R2=0.37, p=0.02).
Discussion and Conclusions
In the acute phase after spinal cord injury, DDE substantially outperforms DTI in predicting chronic functional outcome. However, DTI measurements during the chronic stages of injury, particularly RD, show a much stronger relationship to functional status. The strong relationship between RD and ED1 staining for activated macrophages suggests that inflammation has a pronounced effect on DWI, but similar associations with axonal injury and RD also highlight the complex interpretation of DWI in pathology. Nonetheless, the sensitivity and prognostic benefit of acutely-measured DDE during the acute period following injury, combined with its relative ease in acquisition and analysis, has a strong potential to be used as a noninvasive marker of acute SCI in the clinical setting.Acknowledgements
Project was partially funded through the Research and Education Initiative Fund, a component of the Advancing a Healthier Wisconsin endowment at the Medical College of Wisconsin, the Craig H. Neilsen Foundation, and the Department of Veterans Affairs. NS is a member of the Medical Scientist Training Program at MCW, which is partially supported by a training grant from NIGMS T32-GM080202. NS is partially supported by the National Center for Advancing Translational Science, National Institutes of Health, through grant numbers UL1TR001436 and 1TL1TR001437. Support from the Bryon Riesch Paralysis Foundation is gratefully acknowledged.References
1. I Medana and M Esiri. Axonal damage: a key
predictor of outcome in human CNS diseases. Brain. 2003; 126(Pt 3): 515-530.
2. O Hausmann. Post-traumatic Inflammation
following Spinal Cord Injury. Spinal Cord. 2003; 41:369-378.
3. N Skinner, S Kurpad, B Schmit, et al. Rapid In
Vivo Detection of Rat Spinal Cord Injury with Double-Diffusion-Encoded Magnetic
Resonance Spectroscopy. Magn Reson Med. In Press.
4. J Cohen-Adad, M De Leener, S Benhamou, et al. Spinal
Cord Toolbox: an open-source framework for processing spinal cord MRI data.
Proc 20th Annual Meeting of OHBM, Hamburg, Germany. 2014.
5. M Jenkinson, C Beckmann, T Behrens, et al. FSL. Neuroimage.
2012; 62:782-790.
6. D Basso, M Beattie, and J Bresnahan. Graded
Histological and Locomotor Outcomes after Spinal Cord Contusion Using the NYU
Weight-Drop Device versus Transection. Exp Neurol. 1996; 139: 244-256.
7. P Popovich, P Wei, and B Stokes. Cellular
Inflammatory Response after Spinal Cord Injury in Sprague-Dawley and Lewis
Rats. J Comp Neurol. 1997; 377:443-464.
Figures