0597
Use of Pharmacological MRI (phMRI) to understand the mechanisms leading to convergent procognitive effects of 5-HT6 serotonergic receptors agonist (EMD-386088) and antagonist (SB-271046).1Biomedical MRI group, KU Leuven, Leuven, Belgium, 2GMPc (Groupe Mémoire et Plasticité comportementale), University of Caen-Normandie, Caen, France, 3CNRS UMS3408, GIP CYCERON, Caen, France
Synopsis
Either blocking or activating 5HT6 receptors (5-HT6R), respectively with antagonists or agonists, exert beneficial cognitive effects. Through phMRI, we demonstrated for the first time the similarities and discrepancies in the brain activation induced by an agonist and an antagonist of 5-HT6R. Both drugs similarly activate cortices and hippocampus. SB-271046 positively activates a network including the medio-dorsal raphe while EMD-386088 activates the rostral dorsal raphe. The different patterns of activation elicited in brain regions such as the habenula and the MG/DG nuclei (for SB-271046), or the amygdala and substantia nigra (for EMD-386088) supports different interactions with polysynaptic pathways.
Purpose
5-HT6R is the most recently discovered serotonin receptor and is almost exclusively present in the brain1. Because of its distribution throughout the striatum, nucleus accumbens, cortex and hippocampus2 and the interaction with cholinergic, dopaminergic and/or glutamatergic systems3,4, it has been suggested that it may play a major role in the mechanisms involved in addiction, anxiety, depression and cognitive disorders5, 6. Indeed, blockade of 5-HT6R increases the duration of memory trace and reverses pharmacologically-induced and age-related memory deficits5,7,8. Recent studies demonstrated that 5-HT6R agonists also produces procognitive effects9-11 . The mechanisms by which an agonist and an antagonist could have similar behavioral effects are however unknown. By this study, we aimed to investigate the brain activation induced by specific 5-HT6R antagonist (SB-271046) and agonist (EMD-386088) in order to decipher the mechanisms leading to such a paradoxical effect.Methods
Thirty seven male Wistar rats (2 months old) were randomly divided into 4 groups receiving either NaCl, SB-271046 (1 or 10 mg/kg), or EMD-386088 (5 mg/kg). Pharmacological MRI (phMRI) data were acquired on a 7T Bruker Pharmascan. Animals were anesthetized with 1.5-2.5% isoflurane in 30/70% O2/N2O and placed in MRI-compatible bed. Data were acquired through a surface coil decoupled to a volume transmit coil (72mm internal diameter). Anatomical scans (T2w-RARE, TEeff/TR:70/5000ms, FOV:35x35 mm, matrix:256x256, 15 contiguous slices of 1mm thickness, 3 averages) were acquired then followed by functional scans (T2*w-EPI, TE/TR:19/3000ms, FOV:35x35mm, matrix:128x128, 30 contiguous slices of 0.5mm thickness, 900 repetitions) during which pharmacological challenge was administered after 15 min. Processing was performed using FSL 5.0.8. After preprocessing steps (motion correction, brain segmentation, registration to rat brain template12, slice timing correction, spatial smoothing (Gaussian kernel of FWHM=6mm), mean intensity normalization, FILM prewhitening and temporal high pass filtering), a customized GLM model was computed for SB-271046 and EMD-386088 based on the averaged time-course of all significantly activated voxels for the 30min post-injection. Group mean responses and differences (two-sample unpaired t-test) were computed at the higher level with fixed effect. Z (Gaussianised T/F) statistic images were thresholded using clusters determined by Z > 4 and a (corrected) cluster significance threshold of p = 0.001.Results
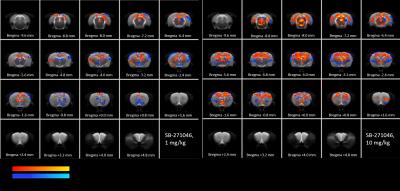
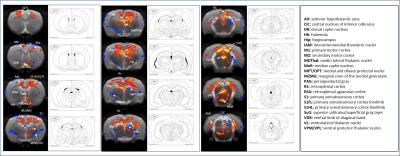
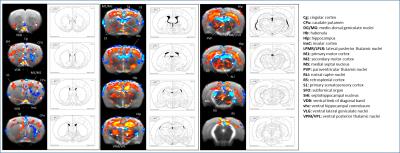
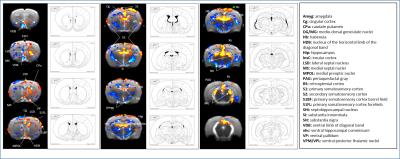
SB-271046 induced a dose-dependent brain activation (Fig.1) with positive BOLD changes principally located in cortical projection areas (motor, somatosensory and retrosplenial) as well as in hippocampus (Hip), mediolateral and mediodorsal thalamic nuclei (MDThal), habenula (Hb) and medio-dorsal raphe nuclei. Negative BOLD changes were observed in ventral posterior thalamic nuclei and medial and olivary pretectal nuclei (MPT/OPT) (Fig.2). EMD-386088 induced a more potent and widespread brain activation with positive BOLD changes in deep layers of motor, somatosensory (M1, M2, S1) and restrosplenial (RS) cortices, hippocampus (Hip), vertical limb of diagonal band of Broca (VDB), ventral thalamus (VPM/VPL) and rostral dorsal raphe nucleus (RLi). Negative BOLD changes were evidenced in cingulate and secondary somatosensory cortices (Cg and S2), ventral hippocampal commissure (vhc), septohippocampal nucleus (SHi), left caudate putamen (CPu), Hb and medio-dorsal geniculate nuclei (MG and DG) (Fig.3). The group comparison of brain activations induced by SB-271046 and EMD-386088 demonstrated significant differences (Fig.4).Discussion
We demonstrate for the first time the brain activation patterns induced by an agonist and an antagonist of 5HT6R. Firstly, we observed that both drugs elicit similar activation in regions such as the hippocampus and cortices. Such a parallel activation in brain regions known to be involved in memory processes supports the similar procognitive effects reported in rodents. Secondly, our results showed discrepancies between the brain activation patterns of SB-271046 and EMD-386088. Indeed, SB-271046 led to an activation in cingular and retrosplenial cortices while EMD-386088 induced negative BOLD changes in these areas. Moreover, SB-271046 was found to activate the medio-dorsal raphe nucleus whereas EMD-386088 induced a specific activation in the rostral dorsal raphe nucleus. Other differences were also highlighted in the CPu, ventral pallidum (VP), VDB, amygdala, VPM/VPL thalamic nuclei and substantia nigra (SN). These differences suggest that the 5-HT6R blockade with SB-271046 and the corresponding stimulation with EMD-386088 activate brain areas involved in memory processes through the recruitment of different networks.Conclusion
Through phMRI, we demonstrated for the first time the similarities and discrepancies in the brain activation induced by an agonist and an antagonist of 5-HT6R. While both drugs similarly activate cortices and hippocampus, SB-271046 and EMD-386088 activate differential networks including the medio-dorsal raphe and the rostral dorsal raphe nuclei, respectively. Moreover, the different patterns of activation elicited by the two drugs in brain regions such as the habenula and the MG/DG nuclei (for SB-271046), or the amygdala and substantia nigra (for EMD-386088) supports different interactions with polysynaptic pathways.Acknowledgements
Rachel Asselot was funded by the French Ministry of Research. Part of the experiments was funded by ERA-NET PrioMedChild program and Regional Council of Normandy.References
1. Ruat M, Traiffort E, Arrang JM, Tardivel-Lacombe J, Diaz J, Leurs R, Schwartz JC. A novel rat serotonin (5-HT6) receptor: molecular cloning, localization and stimulation of cAMP accumulation. Biochem Biophys Res Commun. 1993 May 28;193(1):268-76.
2. Roberts JC, Reavill C, East SZ, Harrison PJ, Patel S, Routledge C, Leslie RA. The distribution of 5-HT(6) receptors in rat brain: an autoradiographic binding study using the radiolabelled 5-HT(6) receptor antagonist [(125)I]SB-258585. Brain Res. 2002 Apr 26; 934(1):49-57.
3. Da Silva Costa-Aze V, Quiedeville A, Boulouard M, Dauphin F. 5-HT6 receptor blockade differentially affects scopolamine-induced deficits of working memory, recognition memory and aversive learning in mice. Psychopharmacology (Berl). 2012 Jul;222(1):99-115.
4. Asselot R, Simon-O'Brien E, Lebourgeois S, Nee G, Delaunay V, Duchatelle P, Bouet V, Dauphin F. Time-dependent impact of glutamatergic modulators on the promnesiant effect of 5-HT6R blockade on mice recognition memory. Pharmacol Res. 2016 Jun 30. pii: S1043-6618(16)30558-8.
5. Quiedeville A, Boulouard M, Da Silva Costa-Aze V, Dauphin F, Bouet V, Freret T. 5-HT6 receptor antagonists as treatment for age-related cognitive decline. Rev Neurosci. 2014;25(3):417-27.
6. Wilkinson D, Windfeld K, Colding-Jørgensen E. Safety and efficacy of idalopirdine, a 5-HT6 receptor antagonist, in patients with moderate Alzheimer's disease (LADDER): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2014 Nov;13(11):1092-9.
7. Da Silva Costa V, Duchatelle P, Boulouard M, Dauphin F. Selective 5-HT6 receptor blockade improves spatial recognition memory and reverses age-related deficits in spatial recognition memory in the mouse. Neuropsychopharmacology. 2009 Jan;34(2):488-500.
8. Da Silva Costa-Aze V, Dauphin F, Boulouard M. Serotonin 5-HT6 receptor blockade reverses the age-related deficits of recognition memory and working memory in mice. Behav Brain Res. 2011 Sep 12;222(1):134-40.
9. Kendall I, Slotten HA, Codony X, Burgueño J, Pauwels PJ, Vela JM, Fone KC. E-6801, a 5-HT6 receptor agonist, improves recognition memory by combined modulation of cholinergic and glutamatergic neurotransmission in the rat. Psychopharmacology (Berl). 2011 Feb;213(2-3):413-30.
10. Pereira M, Martynhak BJ, Andreatini R, Svenningsson P. 5-HT6 receptor agonism facilitates emotional learning. Front Pharmacol. 2015 Sep 16;6:200.
11. Karila D, Freret T, Bouet V, Boulouard M, Dallemagne P, Rochais C. Therapeutic Potential of 5-HT6 Receptor Agonists. J Med Chem. 2015 Oct 22; 58(20):7901-12.
12. Schwarz AJ, Danckaert A, Reese T, Gozzi A, Paxinos G, Watson C, Merlo-Pich E, Bifone A . A stereotaxic MRI template set for the rat brain with tissue class distribution maps and co-registered anatomical atlas: application to pharmacological MRI. NeuroImage 2006, 32(2) 538-550.
Figures