0596
MRI visualization of brain-like tissue formation following implantation of neural precursors into in cerebrospinal fluidNikorn Pothayee1, Dragan Maric2, Kathryn Sharer1, Jung-Hwa Tao-Cheng 3, Stephen Dodd1, Alec Calac1, James Pickel4, and Alan Koretsky1
1Laboratory of Functional and Molecular Imaging, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, United States, 2Flow Cytometry Core Facility, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, United States, 3Electron Microscopy Facility, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, United States, 4Transgenic Core Facility, National Institute of Mental Health, National Institutes of Health, Bethesda, MD, United States
Synopsis
Neural stem cell transplantation has been hailed as a promising approach for treatment of neurological diseases. While most in vivo studies have implanted cells into specific sites in brain tissue, little is known whether the cerebrospinal fluid (CSF) provides a permissive environment in cultivating tissue growth. Here, using MRI, we investigate whether early neural precursor cells could initiate a large-scale formation of new brain tissue in the CSF of adult rat.
Introduction
There is immense interest in transplanting neural precursors as treatment for a number of neurological disorders and stroke. Progress in neural stem cell research has advanced to the critical step in which complex neural tissue structure or “organoid” can be formed in vitro.1 These 3-dimensional (3D) organoids have provided a great tool for understanding central nervous system (CNS) development and disease mechanisms.2,3 However, efforts to form complex neural tissue in vivo are still lacking. Thus far, most studies have transplanted cells into specific sites in brain tissue and commonly shown decreased graft survival. CSF is a key player in supporting development and maintaining homeostasis of CNS.4 Little is known, however, with regard to permissibility of CSF environment to support formation a complex tissue in adult brain. In this study, we investigated whether implantation of early neural precursor cells into a lateral ventricle of the rat brain can produce formation of complex neural tissue within a brain CS.Methods
Embryonic day 14 green
fluorescent protein (GFP) embryos from Lewis rats were isolated and
telencephalon region of developing cortical tissues were carefully dissected
under magnifying scope. The tissues were dissociated using papain-based enzyme
solution. Fluorescence activated cell sorting (FACS) was used to remove
lineage-committed precursors from the uncommitted neural precursor cells
according to a previously published method.5 A 5 microliter cell
suspension at a concentration of 50,000 cells/uL in neurobasal medium
supplemented with growth factors (bFGF and EGF, 20 ng/mL) was stereotaxically
injected into the lateral ventricle of 21- to 24-day-old Lewis rats (40-60 gram
body weights) using the following coordinates: +1.5-1.6 AP and +1.5-1.6 ML from
and – 4.0 DV from bregma. MRI was performed following implantation to monitor a
formation of new tissue in the brain ventricle. All MRI experiments were done
on the 11.7 T animal MRI system (30 cm 11.7 T horizontal magnet, Magnex
Scientific, Oxford, England; MRI Electronics, Bruker Biospin, Billerica, MA).
The following parameters were used: fields of view (FOV) = 1.92 cm2,
matrix size 2563 (100 μm isotropic resolution), 12.5 kHz bandwidth,
TE = 8 ms, TR = 25 ms, and flip angle = 8°. (100 μm isotropic resolution), 12.5
kHz bandwidth, TE 8 ms, and TR 25 ms. MRI was performed 1, 2, 4, 8, and 16
weeks following implantation. Volumes of the new tissues were calculated from
serial VOIs using MIPAV(www.mipav.cit.nih.gov).Results
Serial MRI showed that early neural precursor cells proliferated and expanded over a period of weeks to form tissue structures that occupy the CSF space. These cells expanded several fold and the rapid growth during the first 4 weeks post-implantation followed by a gradual decline in growth rate beyond 8 weeks post-implantation, after which, the tissue showed no significant increase in size (Figure 1A-D). In all, final tissue volume estimated from MRI results was approximately 70 mm3. Gadolinium-enhanced MRI further showed that the new tissue had intact vasculatures that are not permeable to small molecule contrast agents and suggested the presence of intact blood brain barrier (Fig 2A-H). Immunohistology indicated that the new tissues contained fully differentiated neuronal and glial phenotypes that are typically derived from early cortical neural precursor cells during normal brain development. The host brain supplied all microglia, oligodendrocytes, interneurons, and some astrocytes to the newly formed tissue (Figure 3A-B).Discussion
Early neural precursor cells isolated from embryonic cortical region can recapitulate their seminal properties and form neural tissues comprised of cells from within their cortical lineages and differentiated phenotypes. The new tissue that formed in the ventricles integrated into the host brain without noticeable adverse effects on the animal health and behavior. Using MRI, we were able to assess the growth kinetic and blood brain barrier permeability of the newly formed tissue. MRI results predicted that the new tissue was not tumorigenic, which was confirmed by immunohistological and electron microscopy examinations. These findings showed that early-stage neural precursor cells can maintain their intrinsic developmental properties in the adult CNS.Conclusion
This study reported a large-scale expansion of neural precursors following implantation in intra-cerebrospinal fluid. The new-formed tissue elicited the host brain responses that included vascularization and migration of interneurons and glial cells into the tissue. The new tissue integrated into the host brain and formed long-range projection into the host and vice-versa (data not shown). Our results may provide new basis for developing alternative approaches to brain tissue regeneration and implicate an important role of the CSF as an environment to support the growth of neural precursor cells.Acknowledgements
This research was supported by the NINDS Intramural Research Program of National Institutes of Health.References
[1] Lancaster MA, Renner M, Martin C-M et al., Nature 2013;501:373-379. [2] Camp JG Badsha F, Florio M et al., PNAS 2015;112:15672-15677. [3] Clevers H., Cell 2016;165:1586-1597. [4] Redzics ZB, Preston JE, Duncan JA et al., Current Topics in Developmental Biology 2005;71:1-52. [5] Maric D, Pla AF, Chang YH et al., J Neurosci 2007;27:1836-1852.Figures
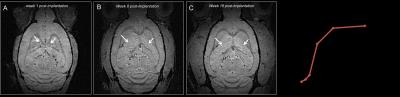
Figure 1.
MRI detection of new tissue formation (A-B) serial MRI revealed growth of new
neural tissue (arrows) following cell implantation into CSF (D) Growth kinetic
obtained from MRI.
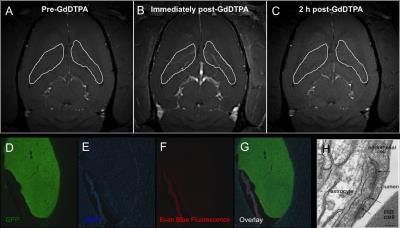
Figure
2. Charactization of blood vessel permeability. (A-C) Gadoliium-enhance MRI
showed no accumulation of GdDTPA (Magnevist) in the new tissue (white ROIs).
(D-G) Evan-blue assay showed lack of evan blue-albumin complexes within the new
tissue. (H) Electron microscopy confirmed the presence of normal BBB tight
junction.
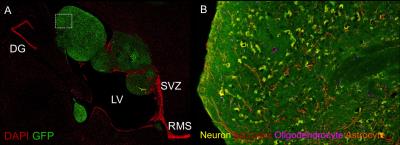
Figure 3. (A) Fluorescence image of new
tissue (GFP+, green) within the ventricles after 8 weeks post-implantation. (B)
Immuno-staining revealed new tissue contained all brain cells phenotypes and was
not tumorigenic.