0595
Can mice outrun the deleterious impacts of radiation to the brain?1Neurosciences and Mental Health, Hospital for Sick Children, Toronto, ON, Canada, 2Mouse Imaging Centre, Hospital for Sick Children, Toronto, ON, Canada, 3Ontario Institute for Cancer Research, Toronto, ON, Canada, 4Department of Medical Biophysics, University of Toronto, Toronto, ON, Canada, 5Department of Psychology, University of Toronto, Toronto, ON, Canada
Synopsis
Pediatric cancer patients who receive cranial radiation therapy (CRT) exhibit cognitive deficits later in life. These deficits are often accompanied by brain structure abnormalities, especially prevalent in the white matter and hippocampus. The objective of this study was to explore the potential of physical exercise to mitigate some of the deleterious effects of CRT on the brain, using a mouse model and high-resolution MRI as a measure of brain structure. We found that irradiated mice housed in cages with access to running wheels showed a remarkable recovery of a number of CRT-induced brain volume deficits, most notably in the hippocampus.
Introduction
Cranial radiation therapy (CRT) in pediatric cancer patients
is associated with cognitive deficits later in life, commonly known as late
effects1-3. Imaging studies in both mice and humans have shown that
CRT leads to impaired brain development, affecting both white and gray matter
areas, particularly regions dependent on ongoing neurogenesis4-8.
Consequently, there is need for interventions that mitigate the deleterious
effects of CRT on the brain. Previous work demonstrated that voluntary running
can rescue decline in neurogenesis of adult mice following CRT9, and
a recent study in long-term pediatric brain tumor survivors who had received
CRT found positive brain changes after completion of an exercise training
program10. We used in vivo anatomical MRI to assess exercise-induced
neuroanatomical changes in young mice following CRT, applied at a stage
approximately equivalent to early childhood. We showed that running ameliorated,
previously well-characterized4-6, post-CRT volume and growth
deficits. There were particularly strong effects in the hippocampus, a brain
region critical for long-term memory formation.
Introduction
Methods
In vivo images were acquired using a T1-weighted gradient echo sequence (TE/TR=8/26 ms, FA=23°, FOV=25x22x22mm, NA=2, scan duration=59 min, “cylindrical” acquisition) with 75 μm isotropic resolution, acquired four mice at a time with 4 cryo-coils on a 7 T Bruker MRI. Female CD-1 mice were imaged at P14, then received a 7 Gy dose of radiation (or sham) at P16. Following another MRI scan at P23, the mice were housed in pairs in either standard cages or cages equipped with a running wheel for the remainder of the study. Subsequent MRI was performed at P42 and P63. Mice were anaesthetized using isoflurane and injected intraperitoneally with MnCl2 (0.4 mmol/kg) 24h prior to each scan. Volume changes were computed using deformation-based morphometry (DBM) via a well-established registration pipeline11-13. Images were analyzed voxel-wise and/or registered to a mouse brain atlas to perform structure-wise analysis. Multiple comparisons were corrected using the false discovery rate14.
Results
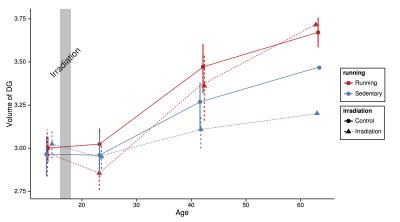
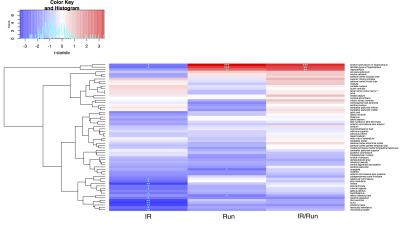
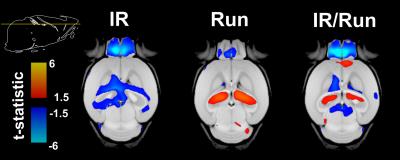
Mice housed in cages with running wheels displayed statistically significant hippocampal volume increases (Fig 1 and Fig 2) in both irradiated (IR/Run) and sham-irradiated groups (Run). IR-treated running mice also showed at least partial recovery of CRT-induced volume losses in the following regions: habenular commissure, stria medullaris, fimbria, stria terminalis, internal capsule, hypothalamus, cerebral aqueduct, third ventricle, fornix, olfactory bulbs, fasciculus retroflexus and mammillary bodies (Fig 2). Interestingly non-irradiated runners (Run) showed statistically significant volume decreases of amygdala and lateral olfactory tract.Discussion
A recent study10 in children investigated the benefits of exercise to long-term pediatric brain tumor survivors. Their findings showed increases in hippocampal volume and improved MR metrics related to white matter health after completing a 3-month long exercise training program. In our mouse study, running also increased hippocampal volume in both irradiated and non-irradiated runners, in addition to decreased CRT-induced volume losses in a number of other regions. These results further strengthen the evidence for an important role of exercise in fostering recovery of structural deficits caused by CRT. In the next steps, MRI and measures of exercise “dose” will be combined to evaluate the relationship between running patterns and anatomical outcomes. In addition, transgenic mice will be used to further investigate mechanisms by which exercise exerts its beneficial effect on the irradiated brain.
Conclusion
While CRT increases survival rates, it also leads to long-term cognitive impairments and neurodegeneration. Unfortunately, there is no cure or standard of care for these treatment-related side effects. We conducted a controlled mouse study that demonstrates that running leads to at least partial mitigation of CRT-induced brain volume loss, with particularly strong benefits for the hippocampus. These results support the idea that exercise promotes neuro-rehabilitation15-16. The potential of exercise for fostering neuro-recovery in children treated for brain tumors with radiation merits further investigation.Acknowledgements
This work was supported by funding from Brain Canada, NeuroDevNet and CBMH Postdoctoral Fellowship (KS), Canadian Institutes for Health Research and Ontario Institute for Cancer Research (BN) and Ontario Institute for Cancer Research grants (BN).References
1. Moxon-Emre I, Taylor MD, Bouffet E, et al. Intellectual Outcome in Molecular Subgroups of Medulloblastoma. J Clin Oncol. 2016;Epub ahead of print.
2. Mabbott DJ, Penkman L, Witol A, et al. Core neurocognitive functions in children treated for posterior fossa tumors. Neuropsychology. 2008;22(2):159-68.
3. Mulhern RK, Butler RW. Neurocognitive sequelae of childhood cancers and their treatment. Pediatr Rehabil. 2004;7(1):1-14
4. Nieman BJ, de Guzman AE, Gazdzinski LM, et al. White and gray matter abnormalities after cranial radiation in children and mice. Int J Radiat Oncol Biol Phys. 2015;93(4):882-91.
5. de Guzman AE, Gazdzinski LM, et al. Treatment age, dose and sex determine neuroanatomical outcome in irradiated juvenile mice. Radiat Res. 2015;183(5):541-9.
6. Gazdzinski LM, Cormier K, Lu FG, et al. Radiation-induced alterations in mouse brain development characterized by magnetic resonance imaging. Int J Radiat Oncol Biol Phys. 2012;84(5):631-8.
7. Mabbott DJ, Noseworthy MD, Bouffet E, et al. Diffusion tensor imaging of white matter after cranial radiation in children for medulloblastoma: correlation with IQ. Neuro Oncol. 2006;8(3):244-52.
8. Nagel BJ, Palmer SL, Reddick WE, et al. Abnormal hippocampal development in children with medulloblastoma treated with risk-adapted irradiation. AJNR Am J Neuroradiol. 2004;25(9):1575-82.
9. Naylor AS, Bull C, Nilsson MK, et al. Voluntary running rescues adult hippocampal neurogenesis after irradiation of the young mouse brain. Proc Natl Acad Sci U S A. 2008;105(38):14632-7.
10. Riggs L, Piscione J, Laughlin S, Cunningham T, et al. Exercise training for neural recovery in a restricted sample of pediatric brain tumor survivors: a controlled clinical trial with crossover of training versus no training. Neuro Oncol. 2016;Epub ahead of print.
11. Lerch JP, Sled JG, Henkelman RM. MRI phenotyping of genetically altered mice. Methods Mol Biol. 2011;711:349-61.
12. Nieman BJ, Flenniken AM, Adamson SL, et al. Anatomical phenotyping in the brain and skull of a mutant mouse by magnetic resonance imaging and computed tomography. Physiol Genomics. 2006;24(2):154-62.
13. Friedel, M., van Eede, M.C., Pipitone, J., et al. Pydpiper: a flexible toolkit for constructing novel registration pipelines. Front Neuroinform 2014;8:67.
14. Genovese, C.R., Lazar, N.A., Nichols, T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage 2002;15:870–878
15. Chaddock L, Pontifex MB, Hillman CH, et al. A review of the relation of aerobic fitness and physical activity to brain structure and function in children. J Int Neuropsychol Soc. 2011;17(6):975-85.
16. Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nat Rev Neurosci. 2008;9(1):58-65.
Figures