0593
Remodeling of resting state functional connectivity following thyromimetic induced remyelination in the mouse brain1Department of Radiology, Medical Physics, University Medical Center Freiburg, Freiburg, Germany, 2Spemann Graduate School of Biology and Medicine, Albert-Ludwigs-University of Freiburg, Freiburg, Germany, 3Laboratory of Engineering, Informatics and Imaging, University of Strasbourg, Strasbourg, France, 4Department of Biophysics and Nuclear Medicine, University Hospital Strasbourg, Strasbourg, France
Synopsis
Characterization of the brain network architecture and its alterations in pathologies are essential for a better understanding of mechanisms of action in health and disease and paves the way for the development of targeted therapeutic strategies. In the field of demyelinating disorders, thyromimetic treatment provides a promising remyelinating strategy inducing oligodendrogenesis in vitro. We tested the potential of sobetirome (GC-1), a thyroid hormone analogue, to induce remyelination in vivo in the cuprizone demyelinated mouse model and we investigated its action on the resting state (rsfMRI) mouse brain functional connectivity.
Introduction:
Multiple Sclerosis (MS) is one of the most common neurological disorders, characterized by extensive loss of myelin, axonal damage and inflammation of the central nervous system.1 RsfMRI is a valuable tool to improve knowledge about the pathophysiology of functional impairments observed in MS patients and is widely used to evaluate alterations in brain functional connectivity (FC) during disease and recovery.2 However, for analysis of the mechanisms underlying the development of myelin disorders as well as for the development of new therapeutic strategies, translational studies in mouse models of demyelination, such as the cuprizone (CUPR) mouse model, are of great importance.3 It was shown previously that thyroid hormone (TH) is an effective strategy to induce remyelination in CUPR treated mice. However, side effects include cardiac deficits due to simultaneous action of TH on cardiomyocytes via α-TH receptors (THR).4,5 The existence of thyromimetics opens an alternative approach to induce oligodendrogenesis while minimizing effects on the heart.6,7 This is based on their selective action on ß-THR, abundant in the brain oligodendrocytes, while avoiding binding to α-THR, abundant in the cardiac system. We analyzed the remyelination capacity of GC-1 in CUPR demyelinated mice and show how such treatment modifies large-scale functional neuronal networks compared to demyelinated and control mice.
Methods:
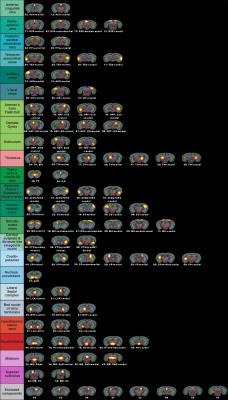
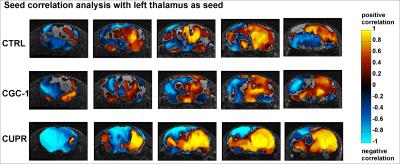
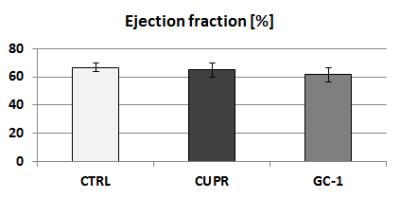
Female 8-week old C57BL/6 mice were treated with 0.2% cuprizone for 12 weeks to induce chronic state of demyelination. This was followed by a 3-week period of GC-1 treatment (daily i.p. injections of 0.15 μg/g body weight) thus using an equimolar dosage similar to TH dosage applied in our previous remyelination study.4 Additionally to this GC-1 group, the setup included a solely demyelinated group (CUPR – 12-week CUPR + 3-week saline treatment) and age matched controls (CTRL – 3-week saline treatment). RsfMRI examinations as well as cardiac MRI were performed at the end of thyromimetic treatment. Data Acquisition: Physiological parameters were monitored and stabilized during imaging performed under moderate medetomidine sedation. MRI was carried-out using a 7 T small bore animal scanner (Biospec 70/20, Bruker, Germany) and a mouse head adapted cryocoil.8 RsfMRI data was acquired using single shot GE-EPI (TE/TR = 10ms/1700ms), 12 axial slices with 0.7mm, FOV 19.2x12mm², planar spatial resolution 0.15x0.15mm2, 200 volumes. Preprocessing was done using SPM8 for motion correction, spatial normalization and alignment with a study based template as well as smoothing (0.4x0.4x1mm³). Data Analysis: Group Independent Component Analysis of rsfMRI data allowed identification of elementary mouse-brain (MB) FC clusters which were assigned to anatomically well-defined brain regions (Fig.1). Their connectional relationship was calculated with partial correlation (PC) providing a comprehensive picture of the MBFC matrices, specific for the 3 experimental groups. We further tested the statistical significant connectional modifications (two-sample t-test, p<0.05) of the brain neural networks between each pair of group. Seed-based correlation analysis was additionally used for a thalamus ROI to test its coherent activity with all other voxels of the brain. Furthermore, cardiac performance was assessed via cardiac MRI to monitor effects of prolonged GC-1 administration focusing on murine left ventricular function.
Results and Discussion:
RsfMRI demonstrated extensive impact of demyelinating pathology on the functional networks of CUPR-treated mice, even at 3 weeks after cuprizone treatment arrest. This is exemplified in Fig.2A showing the significant differences observed between CTRL vs. CUPR, implying that spontaneous remyelination during 3 weeks is not sufficient to compensate for cuprizone induced FC alterations. In contrast, GC-1 treatment considerably reduces the differences induced by cuprizone in FC (Fig.2B), showing significantly less alterations of the MBFC matrix when compared to controls. Moreover, compensatory mechanisms triggered by GC-1 are suggested (while potentially modifying other functional pathways), acting for instance on the default mode network – DMN (see strong changes highlighted in Fig.1C DMN rectangle). Additionally, PC results implied recovery of thalamus FC in GC-1 treated animals. We further present seed-based correlation analysis using a representative ROI of the thalamus (Fig.3), which is a suggested critical 'barometer' of diffuse neuronal pathology and thus ideal region of interest to test effectiveness of new neuroprotective MS drugs.9 Interestingly, this analysis confirms resembling FC patterns for CTRL and GC-1 treated groups and additionally points-out towards increased intra- and inter-thalamic connectivity in CUPR mice. This is a pattern also observed in MS patients.10 Moreover, cardiac MRI results indicated that GC-1 treatment did not impact on left ventricular cardiac function (Fig.4).
Conclusion:
GC-1 remyelinating treatment modulates the MBFC after CUPR induced demyelination. Further work is needed with focus on structural connectivity as well as histopathological analysis to picture probable GC-1 mediated remyelination features to a broader extent.
Acknowledgements
No acknowledgement found.References
1. Confavreux C, Vukusic S. Natural history of multiple sclerosis: a unifying concept. Brain 2006;129:606–616.
2. Bakshi R, Thompson AJ, Rocca MA, Pelletier D, Dousset V, Barkhof F, Inglese M, Guttmann CR, Horsfield MA, Filippi M. MRI in multiple sclerosis: current status and future prospects. Lancet Neurol. 2008;7:615–625.
3. Matsushima GK, Morell P. The Neurotoxicant, Cuprizone, as a Model to Study Demyelination and Remyelination in the Central Nervous System. Brain Pathol. 2001;11:107–116.
4. Harsan L-A, Steibel J, Zaremba A, et al. Recovery from Chronic Demyelination by Thyroid Hormone Therapy: Myelinogenesis Induction and Assessment by Diffusion Tensor Magnetic Resonance Imaging. J. Neurosci. 2008;28:14189–14201.
5. Hübner NS, Merkle A, Jung B, von Elverfeldt D, Harsan L-A. Analysis of left ventricular function of the mouse heart during experimentally induced hyperthyroidism and recovery. NMR Biomed. 2015;28:116–123.
6. Trost SU, Swanson E, Gloss B, et al. The Thyroid Hormone Receptor-β-Selective Agonist GC-1 Differentially Affects Plasma Lipids and Cardiac Activity. Endocrinology 2000;141:3057–3064.
7. Baxi EG, Schott JT, Fairchild AN, Kirby LA, Karani R, Uapinyoying P, Pardo-Villamizar C, Rothstein JR, Bergles DE, Calabresi PA. A selective thyroid hormone β receptor agonist enhances human and rodent oligodendrocyte differentiation. Glia 2014;62:1513–1529.
8. Mechling AE, Hübner NS, Lee H-L, Hennig J, von Elverfeldt D, Harsan L-A. Fine-grained mapping of mouse brain functional connectivity with resting-state fMRI. NeuroImage 2014;96:203–215.
9. Kipp M, Wagenknecht N, Beyer C, Samer S, Wuerfel J, Nikoubashman O. Thalamus pathology in multiple sclerosis: from biology to clinical application. Cell. Mol. Life Sci. 2014;72:1127–1147.
10. Liu Y, Liang P, Duan Y, et al. Altered thalamic functional connectivity in multiple sclerosis. Eur. J. Radiol. 2015;84:703–708.
Figures