0591
Anterograde manganese transport in donor optic nerve following whole eye transplantationYolandi van der Merwe1,2,3, Chiaki Komatsu4, Lin He4, Maxine R Miller3,4, Ian Rosner4, Huamin Tang4, Joel S. Schuman5, Jose-Alain Sahel3, Michael B. Steketee3, Kia M. Washington4, and Kevin C. Chan2,3
1Department of Bioengineering, University of Pittsburgh, Pittsburgh, PA, United States, 2Neuroimaging Laboratory, University of Pittsburgh, Pittsburgh, PA, United States, 3Department of Ophthalmology, University of Pittsburgh, Pittsburgh, PA, United States, 4Department of Surgery, University of Pittsburgh, Pittsburgh, PA, United States, 5Department of Ophthalmology, New York University, New York, NY, United States
Synopsis
Approximately 39 million people worldwide suffer from irreversible blindness. Our recently established whole eye transplant model (WET) gives the opportunity to provide an intact optical system that could restore lost vision. In this study we use manganese-enhanced MRI to examine the anterograde transport of the transplanted and recipient visual pathways following WET. Our results show comparable manganese enhancement between donor and naïve intraorbital optic nerves, suggesting the presence of anterograde manganese transport in the donor optic nerve. This in vivo imaging model system may allow future examinations of neuroregenerative approaches for connecting between the transplanted eye and the recipient’s brain.
Purpose
Approximately 39 million people worldwide are suffering from complete blindness due in part to the inability of the visual system to regenerate after injury1. Recently, we introduced a whole eye transplant (WET) animal model2,3, which gives the opportunity to provide viable retinal ganglion cells and an intact optical system to patients with irreversible vision loss. Using optical coherence tomography and gadolinium-enhanced MRI, we showed that our WET model allows ocular blood circulation, and preserves aqueous humor dynamics as well as blood-ocular and aqueous-vitreous barriers in the transplanted eye4,5. In this study, we aimed to further develop an in vivo imaging model system to determine the functionality of the transplanted and recipient visual pathways following WET. Manganese-enhanced MRI (MEMRI) has been used to monitor anterograde transport in optic nerve injuries6,7,8 and axon regeneration to the visual system9,10,11. Here, we used MEMRI to determine the level of anterograde manganese transport along the visual pathways following WET.Methods
Animal preparation: Seven adult Lewis rats received orthotopic WET on the right side only, as previously described2,4,5. In brief, the donor flap contained the external ear, a section of temporal bone, skin of the eye lid, all ocular tissues and the intraorbital optic nerve. A similar tissue section was removed in the recipients, with the optic nerve being cut at the base of the globe, and the donor graft was transplanted into the recipient with anastomoses between the carotid arteries and external jugular veins (Fig 1). Intraocular pressure (IOP) was measured before MEMRI using the TonoLab tonometer. MEMRI protocols: Three weeks after WET, animals received bilateral intravitreal injections of 1.5µL of 100mM manganese chloride (MnCl2) solution. Before and 1 day after MnCl2 injections, all animals were scanned using fast spin-echo sequences in a 9.4-Tesla/31-cm Varian/Agilent scanner, with a volume transmit and receive coil. 3D isotropic T1-weighted MRI and 2D coronal T1-weighted MRI scans were taken with the following parameters: 3D: FOV= 32x32x32mm3, matrix resolution=192x192x192, TR/TE=200/10.7ms, ETL=8. 2D: FOV= 26x26mm2, matrix resolution=192x192, slice thickness=1mm, slice gap=0.5mm, number of slices=7, TR/TE=600/7.9ms, ETL=8. 2D slices were oriented orthogonal to the prechiasmatic optic nerves (ON). Data analysis: The patterns of manganese enhancement were evaluated qualitatively on 3D MEMRI after axial maximum intensity projection. Quantitatively, regions-of interests (ROI) were drawn manually on the intraorbital ON using 3D scans, and on the prechiasmatic ON, lateral geniculate nucleus (LGN) and superior colliculus (SC) using 2D scans in both hemispheres. An additional ROI was drawn on a nearby saline phantom. The signal intensity for each ROI was measured using ImageJ, and ON, LGN, and SC values were normalized to the phantom. Two-way ANOVA was performed followed by post-hoc multiple comparisons correction tests using GraphPad PRISM.Results
There was no significant IOP difference between naïve and transplanted eyes (p=0.09)(Fig 2). Along the visual pathway projected from the naïve, left eye, significant signal increase was observed post-Mn injection in the left intraorbital and prechiasmatic ON (Figs 3-5), and in the right LGN and SC compared to pre-Mn injection (Figs 3-5)(p<0.0001). Along the visual pathway projected from the transplanted, right eye, significant signal increase was observed post-Mn injection in the right donor intraorbital ON compared to pre-Mn injection (p<0.001)(Figs 3 and 5C). No apparent signal difference was observed between naïve and transplanted intraorbital ON post-Mn injection (p=0.26)(Figs 5A and 5C). No apparent signal intensity difference was observed between pre- and post-Mn injection in the right recipient prechiasmatic ON, left LGN or left SC (p>0.05)(Figs 3-5). Note also the stronger signal enhancement in the transplanted eye relative to the naïve eye (Fig 3).Discussion
The significant signal enhancement in the right intraorbital ON indicates the presence of anterograde manganese transport in the donor ON at 3 weeks after WET. Such enhancement was not statistically different from the left naïve ON, suggestive of comparable extents of anterograde transport between donor and naïve ON. No enhancement was detected in the right prechiasmatic ON, left SC or left LGN of the recipient’s brain, suggesting a discontinuation of anterograde manganese transport across the nerve coaptation site after ON transection and apposition. Such observation coincided with the less manganese clearance from the transplanted eye.Conclusions
With comparable IOP between transplanted and naïve eyes, MEMRI suggested the presence of anterograde manganese transport in the donor ON to a comparable extent as the opposite naïve ON at 3 weeks after WET. Future MEMRI studies will examine neuroregenerative approaches12,13 for connecting between the transplanted eye and the recipient’s brain, so as to regain the neuronal structure and function of the current WET model.Acknowledgements
This work was supported by the The Office of the Assistant Secretary of Defense for Health Affairs under Award No. W81XWH-14-1-0421, VA Pittsburgh Healthcare Administation, National Institutes of Health P30-EY008098 (Bethesda, Maryland); Eye and Ear Foundation (Pittsburgh, Pennsylvania); and Research to Prevent Blindness (New York, New York).References
[1] World Health Organization, www.who.int/blindness/data_maps. Accessed October 5th, 2016.[2] Washington, KM., et al. Viability, structural integrity, and aqueous humor dynamics are established in an orthotopic whole eye transplant model. Investigative Ophthalmology & Visual Science. 2015; 56 (7), 4757.
[3] Couzin-Frankel, J. Second Sight. Science. March 2015; 347(6227):1194-7.
[4] Van der Merwe Y, et al. In vivo evaluation of ocular physiology and structural integrity of the optic nerve upon whole eye transplantation using gadolinium-enhanced MRI and diffusion tensor imaging. ISMRM 2015 Annual Meeting Conference Proceedings, Toronto, Canada, May 2015.
[5] Washington, KM., et al. Studies of structure and function in whole eye transplantation. Investigative Ophthalmology & Visual Science. 2016; 57 (12).
[6] Thuen, M., et al. Manganese-enhanced MRI of the optic visual pathway and optic nerve injury in adult rats. Journal of magnetic resonance imaging. Oct 2005; 22(4):492-500.
[7] Chan, KC., et al. Evaluation of the retina and optic nerve in a rat model of chronic glaucoma using in vivo manganese-enhanced magnetic resonance imaging. Neuroimage. April 2008; 40(3):1166-1174.
[8] Chan, KC., et al. In vivo retinotopic mapping of superior colliculus using manganese-enhanced magnetic resonance imaging. Neuroimage. January 2011; 54(1):389-395.
[9] Sandvig A., et al. Axonal tracing of the normal and regenerating visual pathway of mouse, rat, frog, and fish using manganese-enhanced MRI (MEMRI). Journal of magnetic resonance imaging. Sep 2011; 34(3):670-5.
[10] Thuen, M., et al. Combination of Mn(2+)-enhanced and diffusion tensor MR imaging gives complimentary information about injury and regeneration in the adult rat optic nerve. Journal of magnetic resonance imaging. Jan 2009; 29(1):39-51. [11] Liang, Y., et al. CNS regeneration after chronic injury using a self-assembled nanomaterial and MEMRI for real-time in vivo monitoring. Nanomedicine: Nanotechnology, biology, and medicine. June 2011; 7(3):351-359.
[12] Moore, DL., and Goldberg JL. Four steps to optic regeneration. Journal of Neuro-ophthalmology. Dec 2010;30(4):347-360.
[13] Benowitz, LI., et al. Reaching the brain: Advances in optic nerve regeneration. Experimental neurology. Dec 2015; (15)30141-2.
Figures
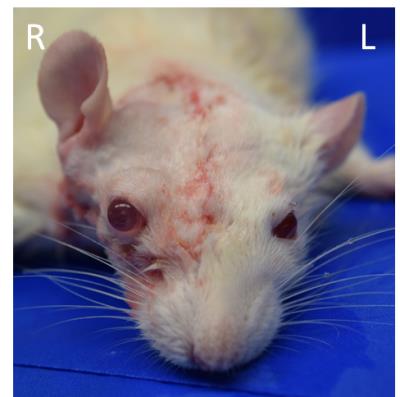
Figure 1. A
picture of the recipient rat after whole eye transplantation to the right side.
Note the comparable color in the eyes and ears between both sides, indicative
of restoration of blood circulation.
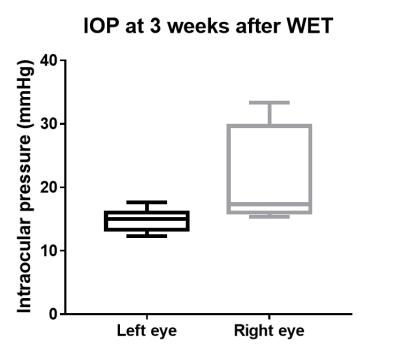
Figure
2. Intraocular
pressure (IOP) at 3 weeks after WET. There was no significant difference in IOP
between the left, naïve and right, transplanted eyes (Two-tailed, paired
t-test, p=0.09). Box plots show minimum, first quartile, median, third
quartile, and maximum.
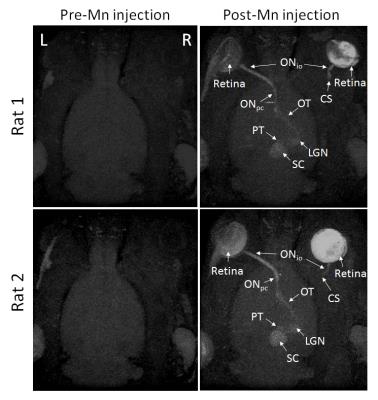
Figure 3. Axial
maximum intensity projection (MIP) of 3D MEMRI from 2 representative WET
animals. (Left column) MIP pre-Mn injection. (Right column) MIP post-Mn
injection. WET was performed to the right eye. ONio: intraorbital optic
nerve, ONpc: prechiasmatic optic nerve, OT: optic tract, LGN:
lateral geniculate nucleus, PT: pretectum, SC: superior colliculus, CS:
coaptation site in the right, transplanted eye.
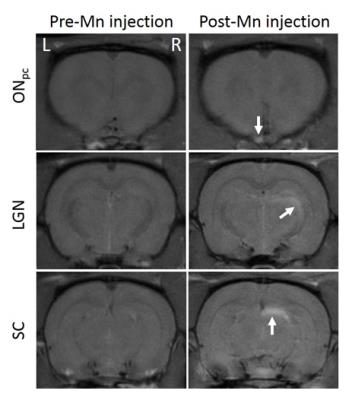
Figure 4.
Representative 2D coronal MEMRI at the levels of the prechiasmatic optic nerve
(ONpc, top row), lateral geniculate nucleus (LGN, middle row) and the
superior colliculus (SC, bottom row) pre- (left column) and post-Mn injection
(right column). Arrows indicate signal enhancement in the left ONpc,
right LGN and right SC projected from the naïve, left eye. No apparent signal
enhancement was observed in the right ONpc, left LGN or left SC projected
from the transplanted, right eye.
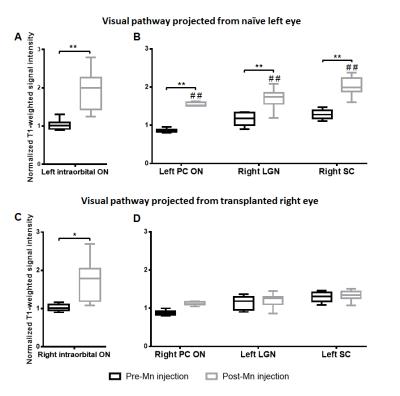
Figure
5.
Normalized T1-weighted signal
intensities of the intraorbital optic nerves (ON) taken from 3D MEMRI scans (A,
C), and the prechiasmatic optic nerve (PC ON), lateral geniculate nucleus
(LGN), and superior colliculus (SC) taken from 2D MEMRI scans (B, D). A and B
show visual pathway projected from naïve, left eye, and C and D shows visual
pathway projected from transplanted, right eye. Post-hoc Sidak’s tests between
pre- and post-Mn injection, *p<0.001, **p<0.0001. Post-hoc Sidak’s tests
between left and right hemispheres, ##p<0.0001. Box plots show
minimum, first quartile, median, third quartile, and maximum.