0588
Dual region-selective spiral pTX excitation for digit mapping fMRI in motor cortex and cerebellum1Faculty of Psychology and Neuroscience, Maastricht University, Maastricht, Netherlands, 2Scannexus BV, Maastricht, Netherlands
Synopsis
We demonstrate the use of 2D-selective excitation with parallel transmission to (a) simultaneously perform BOLD fMRI on two apart brain regions, and (b) benefit from high acceleration factors achievable with sparse volume excitation. Using 8ch pTX at 7T, we apply spatially selective pulses to excite the primary motor and sensory cortices, as well as cerebellum. tSNR measures confirm superior performance compared to whole-brain excitation at the same TR. A finger tapping task is used to elicit digit-specific activation, which is observed in primary cortex and cerebellum.
Purpose
To demonstrate the use of 2D-selective excitation with parallel transmission to (a) simultaneously perform BOLD fMRI on two apart brain regions, and (b) benefit from high acceleration factors achievable with sparse volume excitation.Introduction
The availability of ultra-high field (UHF, ≥7T) enables BOLD fMRI at spatial resolutions well into the sub-millimeter range. A primary challenge is that image acquisition time increases drastically, and in order to keep volume repetition times compatible with the requirements for fMRI (TR<2.5s) the experiment is often restricted to imaging only one part of the brain. In such cases the use of local receiver arrays in combination with careful FoV planning is one common choice. It may moreover be beneficial to exclude parts of the brain that are known to introduce physiological noise, such as the ventricles or brain stem. In practice it therefore becomes challenging to cover two apart brain regions at the same time, for example the motor cortex and cerebellum, without (a) excessive imaging time, (b) large g-fcator nosie amplification and/or (c) inevitable inclusion of noise-introducing anatomy. Prior work has used parallel 2D-selective excitations [1-3] in single-brain regions to work around this limitation, and it has been shown that large TR reductions can be achieved thanks to high parallel acceleration factors in case of sparse excitations [1]. Another study has used (single-channel) 2D selective excitations for simultaneous fMRI in distributed regions at 3T [4].
In this work we extend this approach to the selective excitation of two brain regions with pTX spiral excitation, and perform a BOLD fMRI finger tapping task [5] to elicit digit-specific BOLD activation simultaneously in the primary motor and sensory cortex, as well as the cerebellum.
Methods
Experiments on three volunteers were carried out on a 7T whole-body MR scanner (MAGNETOM, Siemens Healthineers, Erlangen, Germany) with a 8Ch-pTX/32Ch-Rx coil (Nova Medical, Wilmington, MA, USA).
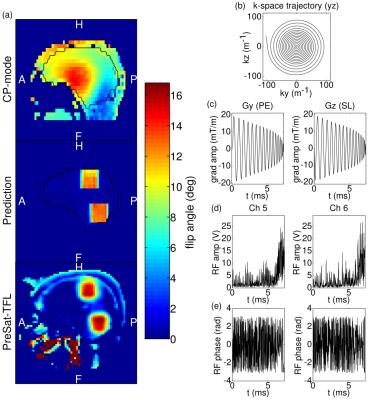
RF pulse design: An in-house 3D EPI sequence [3] was modified to apply externally calculated spatially selective excitation pulses. Target regions were defined by manual drawing of a NIFTI mask that served as input into the magnitude least-squares optimisation [7,8] under the small tip-angle approximation [9]. B0 map obtained by dual-echo 3D GRE [10], B1+ sensitivity maps collected by a transmit phase-encoded [11] T2 and T2* compensated version of DREAM [12] and a spiral-in gradient trajectory [13] (details in Figure 1) in the sagittal plane were combined to form the system matrix used in the RF pulse optimisation according to the spatial domain method [14]. The excitation pattern was verified by PreSat-TFL [15] flip-angle mapping.
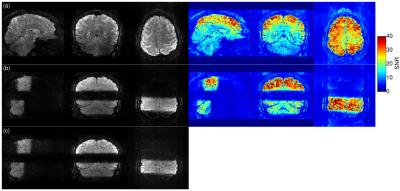
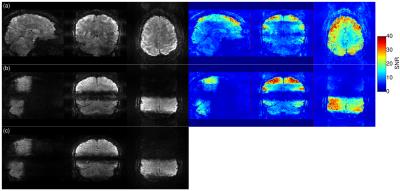
Image acquisition: 3D EPI with 1mm iso voxels (transverse FoV 192x192x144, in-plane GRAPPA-3, 6/8 partial Fourier, TE=18ms, TR=56ms, FA=10deg, BW=1370Hz/px, ESP=0.85ms) were acquired with additional factor-3 and facor-4 through-plane undersampling to yield volume TR(3x3)=2000ms and TR(3x4)=1500ms, respectively. EPI- and FLASH-based GRAPPA reference scans were explored, leading to the choice of FLASH. Image quality was assessed by tSNR over 50 volumes.
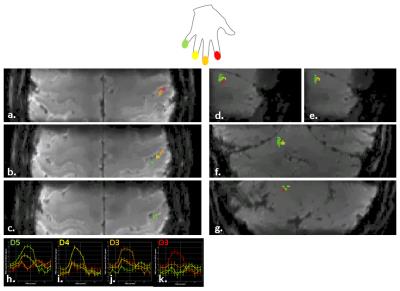
Digit mapping fMRI tasks: Block design similar to [5] with 10s blocks of 1Hz paced tapping per digit (order: index-ring-middle-pinky), separated by 14s of rest; 9 repeats gave total duration of 15min. Analysis was performed in Brainvoyager to identity maps of digit-specific activation. Images were motion-corrected and high-pass filtered but not smoothed temporally or spatially.
Results
Figure 1 provides details on the RF pulse. Good agreement is observed between the predicted and observed spatial selectivity and moreover, unlike in the CP mode, approximately equal flip-angles are achieved in both target regions.
Figure 2 and 3 show image results for the 3x3 and 3x4 protocols with whole-brain (a) and partial (b) excitation. Image quality with FLASH calibration scan ((c) vs (d)) is superior. As expected and in agreement with [1] the tSNR in case of whole brain excitation (top right) is much lower than with selective excitations (bottom right).
Figure 4 shows one subject’s results from the digit mapping task (single 15min run), for details see caption.
Conclusion
High-resolution fMRI was simultaneously performed in two selectively excited brain regions. The high acceleration factors permitted short TR that is compatible with the requirements for task fMRI, with greatly reduced (t)SNR penalty compared to the full brain excitations at the same TR.Acknowledgements
The authors acknowledge scan time support from the FPN-MBIC and Scannexus development projects.References
1. Mooiweer R, Sbrizzi A, Raaijmakers AJ, van den Berg CA, Luijten PR, Hoogduin H (2016) Combining a reduced field of excitation with SENSE-based parallel imaging for maximum imaging efficiency. Magn Reson Med 10.1002/mrm.26346
2. Vinding MS, Guerin B, Vosegaard T, Nielsen NC (2015) Local SAR, global SAR, and power-constrained large-flip-angle pulses with optimal control and virtual observation points. Magn Reson Med 10.1002/mrm.26086
3. Setsompop K, Wald LL, Alagappan V, Gagoski BA, Adalsteinsson E (2008) Magnitude least squares optimization for parallel radio frequency excitation design demonstrated at 7 Tesla with eight channels. Magn Reson Med 59 (4):908-915
4. Finsterbusch J (2015) Simultaneous functional MRI acquisition of distributed brain regions with high temporal resolution using a 2D-selective radiofrequency excitation. Magn Reson Med 73 (2):683-691
5. van der Zwaag W, Kusters R, Magill A, Gruetter R, Martuzzi R, Blanke O, Marques JP (2013). Digit somatotopy in the human cerebellum: a 7T fMRI study. Neuroimage. 15;67:354-62
6. Poser BA, Koopmans PJ, Witzel T, Wald LL, Barth M. Three dimensional echo-planar imaging at 7 Tesla. Neuroimage 2010;51:261-6.
7. Setsompop K, Wald LL, Alagappan V, Gagoski BA, Adalsteinsson E (2008) Magnitude least squares optimization for parallel radio frequency excitation design demonstrated at 7 Tesla with eight channels. Magn Reson Med 59 (4):908-915
8. Sbrizzi A, Hoogduin H, Lagendijk JJ, Luijten P, G. SGL, van den Berg CAT (2011) Time efficient design of multi dimensional RF pulses: Application of a multi shift CGLS algorithm. Magn Reson Med 66 (3):879-885
9. Pauly J, Nishimura D, Macovski A (1989) A k-space analysis of small-tip-angle excitation. J Magn Reson (1969) 81 (1):43-56
10. Tse DHY, Wiggins CJ, Ivanov D, Brenner D, Hoffmann J, Mirkes C, Shajan G, Scheffler K, Uludag K, Poser BA (2016) Volumetric imaging with homogenised excitation and static field at 9.4 T. Magn Reson Mater Phy 29 (3):333-345
11. Tse DHY, Poole MS, Magill AW, Felder J, Brenner D, Jon Shah N (2014) Encoding methods for B1(+) mapping in parallel transmit systems at ultra high field. J Magn Reson 245:125-132
12. Nehrke K, Versluis MJ, Webb A, Bornert P (2014) Volumetric B1 (+) mapping of the brain at 7T using DREAM. Magn Reson Med 71 (1):246-256
13. Pipe JG and Zwart NR (2014) Spiral trajectory design: A flexible numerical algorithm and base analytical equations. Magn Reson Med 71(1):278-285
14. Grissom W, Yip C-y, Zhang Z, Stenger VA, Fessler JA, Noll DC (2006) Spatial domain method for the design of RF pulses in multicoil parallel excitation. Magn Reson Med 56 (3):620-629
15. Chung S, Kim D, Breton E, Axel L (2010) Rapid B1+ mapping using a preconditioning RF pulse with TurboFLASH readout. Magn Reson Med 64 (2):439-446
Figures