0582
High-resolution Spiral fMRI at 7T1Institute for Biomedical Engineering, ETH Zurich and University of Zurich, Zuerich, Switzerland, 2Translational Neuromodeling Unit, IBT, University of Zurich and ETH Zurich, Zurich, Switzerland, 3Skope Magnetic Resonance Technologies, Zurich, Switzerland, 4Translational Neuromodeling Unit, IBT, University of Zurich and ETH Zurich, Zuerich, Switzerland, 5Wellcome Trust Centre for Neuroimaging, University College London, London, United Kingdom, 6Max Planck Institute for Metabolism Research, Cologne, Germany
Synopsis
High-resolution spiral fMRI acquisition at 7 Tesla is shown with excellent image quality and geometric veracity. Both, high-resolution spiral-out (1mm isotropic) and multi-echo spiral-in/out readouts (1.5mm) are acquired in a visual paradigm, performed by 2 subjects. The datasets are analyzed by SPM and activation patterns are shown overlaid over the actual fMRI datasets of almost structural imaging quality.
Introduction
Single-shot 2D-EPI readouts are the unrivalled acquisition strategy in BOLD fMRI.
From a conceptual point of view though, spiral acquisitions feature a large set of advantages [1] over EPI such as efficient k-space coverage or reduced sensitivity to cardiac-induced pulsatile motion. The short readout times can be translated to higher temporal or spatial resolution, while they mitigate T2*-induced signal decay and static off-resonance effects. Both issues pose a challenge at ultra-high field, where BOLD fMRI is preferably performed due to a significant baseline sensitivity advantage.
In practice, the use of spiral imaging is limited by hardware imperfections such as eddy currents, delays, mechanical vibrations, as well as static B0 off-resonance effects. Ensuing artifacts often degrade image quality to a non-tolerable level.
Recently, impeccable single- and multi-shot spiral imaging at 7T was shown by joint correction for off-resonance and trajectory errors. This progress rests on an expanded signal model, which encompasses simultaneous accounting for measured static and dynamic field perturbations, and can be combined with image reconstruction performed by model inversion using an extension of the iterative non-Cartesian SENSE-algorithm [1,2].
In this work we aim to further explore the potential benefit of spiral acquisition for functional MRI, for high-resolution and multi-echo applications.
Methods
Setup
Two healthy volunteers were scanned on a 7T Achieva system (Philips), using a 1-channel transmit, 32-channel receive coil (Nova Medical). 16 NMR field-probes were placed in optimal positions between the receive and transmit module using a clip mount system and connected to a dedicated MR acquisition system [3]. Visual stimulation was delivered via an LCD visor (VisuaStim).
Sequence and Trajectories
A high-resolution 2D single shot spiral trajectory (undersampling factor R=4, 1mm in-plane resolution) as well as a spiral-in/out trajectory (1.5mm in-plane resolution) were designed, fully exploiting the system’s slew-rate and gradient strength limits [4]. In total, 36 transverse-oblique slices (1mm+0.2mm gap) were acquired covering the visual cortex (FOVz=43mm) for all sequences, with TR=3s. A spin-warp multi-echo sequence (6 echoes, delta TE=1ms) was measured prior to the functional runs with identical geometry, to estimate the sensitivity and off-resonance maps, Concurrent field monitoring was performed in all scans, using NMR field probes. The probes were excited before the start of the spiral trajectories and sampled with 1MHz bandwidth using a dedicated MR acquisition system. MR signals from the head-coil were acquired via the scanner spectrometer.
Signal Processing and Image Reconstruction
For every time point during the imaging readout, 9 k-space coefficients – corresponding to a spherical harmonic basis set up to 2nd order – were computed from the probe signals. To minimize error in the measurements of the encoding dynamic fields, the probe positions were optimized, respecting the geometrical boundaries of the coil. On the coil raw data, an iterative, CG-based image reconstruction was performed (SENSE with multi-frequency interpolation, [2,7]). For the encoding model, the measured field dynamics up to first order (k0 - k3) were used, in combination with the sensitivity- and static off-resonance maps [8,9].
fMRI Paradigm and Analysis
A 5min block paradigm of alternating visual quarter-field stimulation was used to elicit BOLD activation. 5 blocks (15s each) of upper-left/lower-right (ULLR) quarterfield stimulation were interleaved with 5 blocks of upper-right/lower left (URLL) stimulation, and separated by 15s rest blocks. Image realignment and statistical analysis with a general linear model was performed in SPM12. Activation was assessed using F-Maps contrasting ULLR vs URLL blocks.
Results
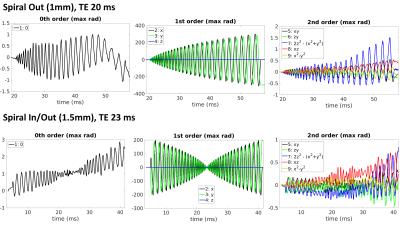
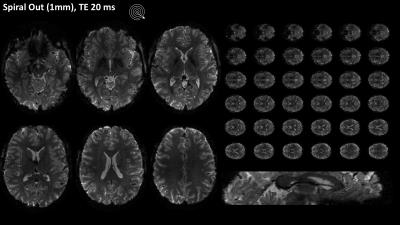
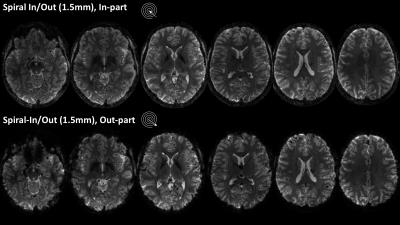
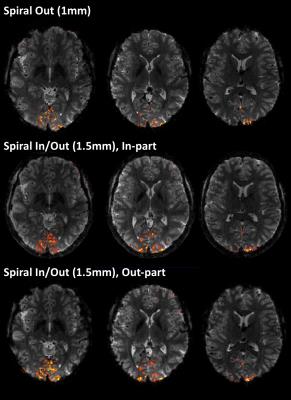
Field evolutions during spiral encoding of up to 40ms were monitored (Fig.1). Combined with static B0 off-resonance maps, high-quality artifact-free single-shot T2* spiral-out images (Fig.2) could be reconstructed, with near-isotropic resolution. At a slightly lower resolution (1.5mm), individually reconstructed in- and out-images of the spiral-in/out readout offered two BOLD-sensitive images within the same acquisition time (Fig.3). The in-part in particular showed high geometric fidelity along the cortex edges and reduced signal dropouts due to lower T2*-weighting of the high spatial frequencies. For all spirals, significant activation of the visual cortex elicited by the block paradigm could be detected (Fig.4). While the high-resolution spiral showed improved specificity, with activation closely following cortex gyrification, the spiral-out part of the in/out trajectory exhibited higher sensitivity to BOLD, reflected in higher F-values.Discussion/Conclusion
We have shown that spiral fMRI is feasible at 7T with high image quality and geometric fidelity. Furthermore, combined spiral-in/out readouts offer distinct BOLD sensitivity and specificity characteristics and can be readily reconstructed with the proposed signal model and reconstruction framework. Optimized single-shot spirals, e.g. using variable density sampling, or multi-echo readouts varying have the potential of further increasing imaging speed and SNR at ultra-high field.Acknowledgements
No acknowledgement found.References
[1] B.J. Wilm et al., Magnetic Resonance in Medicine, 2016, in press.
[2] K.P. Pruessmann et al., Magnetic Resonance in Medicine, 2001, 46, 638?651.
[3] B.E. Dietrich et al., Magnetic Resonance in Medicine, 2016, 75, 1831.
[4] M. Lustig, S.-J. Kim, J.M. Pauly, IEEE Transactions on Medical Imaging, 2008, 27, 866.
[5] C. Barmet et al., in Proc. Intl. Soc. Mag. Reson. Med. 18, 2010, 216.
[6] C. Barmet, N. De Zanche, K.P. Pruessmann, Magnetic Resonance in Medicine, 2008, 60, 187?197.
[7] L.C. Man, J.M. Pauly, A. Macovski, Magnetic Resonance in Medicine, 1997, 37, 785.
[8] B.P. Sutton, D.C. Noll, J.A. Fessler, IEEE Transactions on Medical Imaging, 2003, 22, 178.
[9] C. Barmet, J. Tsao, K.P. Pruessmann, in Proceedings of the ISMRM, 2005, 682.
Figures