0574
Pile-up and Ripple Artifact Correction Near Metallic Implants by Alternating Gradients1Radiology, Stanford University, Stanford, CA, United States, 2Electrical Engineering, Stanford University, Stanford, CA, United States
Synopsis
Multi-Spectral Imaging techniques have been shown to significantly reduce metal-induced artifacts. However, they often suffer from residual pile-up and ripple artifacts in the vicinity of metal, where the metal-induced off-resonance gradient “cancels” the frequency-encoding gradient. Fully phase-encoded methods can overcome the drawbacks of frequency encoding, but usually incur prohibitively long scan times. Here we address this limitation by combining two acquisitions with alternating-sign readout and slice-select gradients. We demonstrate with simulations, phantom and in-vivo scans that the proposed method can effectively suppress pile-up and ripple artifacts, and reduce the signal loss areas near the implants.
Purpose
Multi-Spectral Imaging (MSI) techniques, such as SEMAC1 and MAVRIC-SL2, significantly reduce metal-induced artifacts, but often suffer from pile-up and ripple artifacts in the vicinity of metal where metal-induced off-resonance gradients cancel the readout gradient3,4. Fully phase-encoded methods can overcome this limitation5,6, but usually incur prohibitively long scan times. Techniques including Jacobian-based correction7, slice overlap4 and deblurring2,8 can reduce the intensity fluctuations, but do not recover the lost structural information in pile-up areas. Here we address this limitation by combining two acquisitions with alternating-sign readout and slice-select gradients.Methods
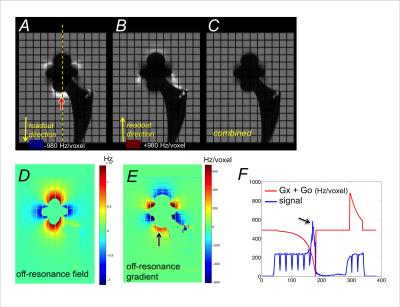
As shown previously3 and in Fig.1 A&B (described in results), pile-ups/ripples appear where the off-resonance gradient $$$G_o$$$ is opposite to the readout gradient $$$G_x$$$. The reduced magnitude of the effective readout gradient $$$(G_o+G_x) $$$ in these areas expands the encoded pixel size, causing irrecoverable loss of resolution in the readout direction. Additive $$$(G_o+G_x)$$$ reduces the encoded pixel size, but its effect can be mostly corrected by deblurring8 and Jacobian correction5. With the readout gradient $$$G_x$$$ inverted, these artifacts appear in different locations, which can be exploited to suppress pile-up/ripple artifacts.
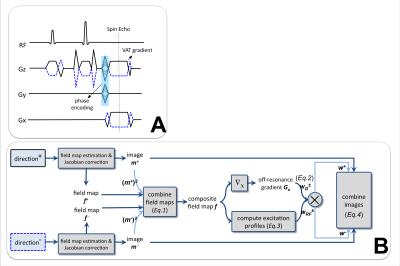
The voxel shearing effect caused by view-angle tilting (VAT), used with selective MSI, can be matched between the two gradient directions by additionally inverting the slice-select and VAT gradients $$$G_z$$$ (Fig.2A), which also changes the regions of non-excited signal loss.
A combination that exploits the different locations of pile-up artifacts and non-excited regions is outlined in Fig.2B:
1. Standard field map estimation, deblurring8 and Jacobian correction5 are performed separately for images from each gradient direction. The resulting field maps and composite images are denoted as $$$f^+,f^-$$$ and $$$m^+,m^-$$$ , where + and - denote the different gradient directions.
2. A composite field map $$$f$$$ is obtained by combining two field maps:
$$f=[(m^+)^2f^++(m^-)^2f^-]/[(m^+)^2+(m^-)^2]\hspace{10mm}(\mathrm{Eq.1})$$
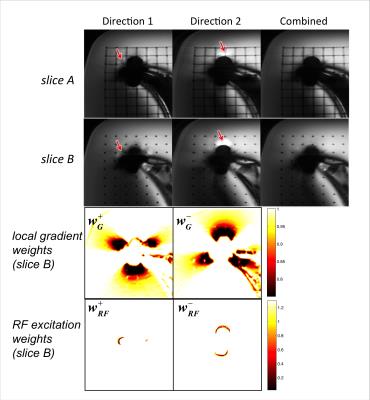
3. The off-resonance gradient $$$G_o$$$ is computed as the finite difference of $$$f$$$ along the readout direction. The local gradient weights of each gradient direction (Fig.3 row3) are computed as
$$w_G^±(x,y,z)=\max\{\min\{1±\frac{G_o(x,y,z)}{G_x},1\},0\}\hspace{10mm}(\mathrm{Eq.2})$$where lower values indicate more severe pile-ups.
4. RF excitation weights (Fig.3 row4) are computed as
$$w_{RF}^±(x,y,z)=\Sigma_b{R_b^2(f(x,y,z)±\gamma\kern-0.6em-G_zz)}\hspace{10mm}(\mathrm{Eq.3})$$
where $$$R_b(\cdot)$$$ represents RF frequency profile of bin b. Lower values indicate non-excited regions.
5. The weighted image combination is
$$f=(w^+m^++w^-m^-)/(w^++w^-)\hspace{10mm}(\mathrm{Eq.4})$$
where the overall weights $$$w^±=\exp\{\alpha w_G^±+\beta w_{RF}^±\}\hspace{2mm}(\mathrm{Eq.5})$$$, scaling factors $$$\alpha=4$$$ and $$$\beta=10$$$ are selected empirically.
The proposed method was validated with simulations with a digital
phantom, and MAVRIC-SL
scans of a hip implant phantom and volunteers with knee and hip implants. All scans were performed on GE 3T MRI systems with 24 spectral bins
spanning ±12kHz, and ±125kHz readout bandwidth. In each scan, the original MAVRIC-SL sequence
was run first, and then repeated with readout, slice-select and VAT gradients all inverted. For volunteer scans, translational registration was performed before
the combination. The final image reconstructed with the proposed method was compared
with the images $$$m^+$$$ and $$$m^-$$$ at step 1.
Results & Discussion
In the simulated acquisition, the proposed combination restored image resolution in areas where pile-up artifacts and blurring were seen in the source acquisitions (Fig.1). The lower signal intensity regions due to additive $$$G_o+G_x$$$, while not reconstructed perfectly, were corrected reasonably well from deblurring and Jacobian correction. Low-signal regions compromised visualization of structures near implants less than pile-up or ripple artifacts.
In the phantom (Fig.3), areas of pile-ups/ripples and non-excited signal loss in images from individual gradient directions were identified by the corresponding combination weights and suppressed in the combined image. The resolution grid in the combined image had the same level of sharpness as each gradient direction’s image, indicating that no additional blurring was introduced by the combination.
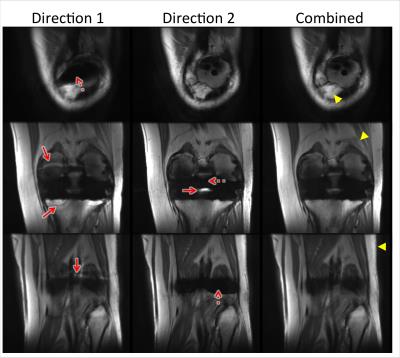
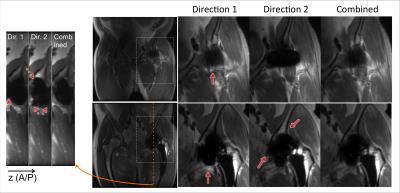
The in-vivo knee and hip results (Figs.4&5) both demonstrated that the proposed combination effectively suppressed the pile-ups/ripples that obscured the underlying structures near the implants. The artifact suppression in combined images was always at least as good as that of either direction, and usually better than both. In these two cases, images of one gradient direction had fewer pile-ups/ripples than the other. But the “better” gradient direction (with smaller artifacts) depends on the specific device/orientation and is unknown before the acquisition, so it cannot be reliably chosen in advance.
A penalty of the proposed method is the doubled scan time. Patient motion during this long scan time may cause blurring in the combined image, and slight blurring of fine structures was observed (arrowheads in Fig.4). In future work, accelerating the acquisitions9-11 will be explored, as well as schemes to interleave acquisitions and exploit redundancies.
Conclusion
We have demonstrated an approach to combine MSI acquisitions with opposing gradients to substantially reduce residual pile-up/ripple artifacts near metal.Acknowledgements
NIH: R01 EB017739, R21 EB019723, P41 EB015891; NSF GRFP: DGE-114747; research support from GE Healthcare.References
[1] Lu et. al, MRM 62:66-76, 2009; [2] Koch et. al, MRM 65:71-82, 2011; [3] Koch et. al, MRM 71: 2024-34, 2014; [4] den Harder et. al, MRM 73: 318-24, 2015; [5] Ramos-Cabrer, MRI 22:1097-103, 2004; [6] Artz et. al, MRM 71:681–690, 2014; [7] Koch et. al, ISMRM 2011, p 3173; [8] Quist et. al, ISMRM 2016, p 4241; [9] Worters et. al, JMRI 37:243–248, 2013; [10] Otazo et, al, MRM Early View, 2016; [11] Shi et. al, ISMRM 2016, p 47.Figures