0567
Characterization of Gd-DOTA-APC, a novel cancer-targeting MRI contrast agent1Medical Physics, University of Wisconsin-Madison, Madison, WI, United States, 2Radiology, University of Wisconsin-Madison, Madison, WI, United States, 3Medical Engineering Group, Morgridge Institute for Research, Madison, WI, United States
Synopsis
In this work we characterize the relaxivity, chemical stability, and tumor-specific uptake of a cancer-targeting T1-shortening contrast agent, Gd-DOTA-APC. We observe striking longitudinal relaxivity of up to 16.5 s-1/mM and 10.6 s-1/mM at 1.5T and 3.0T, respectively. High chemical stability was measured, with less than 0.1% free Gd3+ detected after dissolution of the agent in buffer. Finally, we observe tumor-specific T1-weighted signal enhancement following Gd-DOTA-APC delivery, sustained out to seven days after administration. These observations indicate that Gd-DOTA-APC holds potential as a safe and effective agent for targeted cancer imaging.
Introduction
Alkylphosphocholine (APC) analogs have shown broad-spectrum cancer-specific uptake for PET-based detection of tumors and metastases in a variety of animal models, as well as in human patients1. With a novel labeling technique using the chelator 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetracetic acid (DOTA), we have produced an APC analog labeled with the MR contrast agent Gd3+. The objectives of this work are to characterize the MR relaxation characteristics and stability of Gd-DOTA-APC and to demonstrate tumor-specific uptake in an animal model.Methods
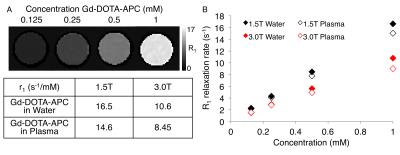
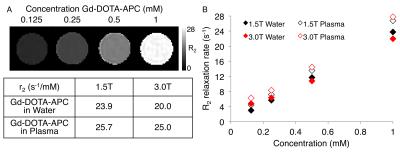
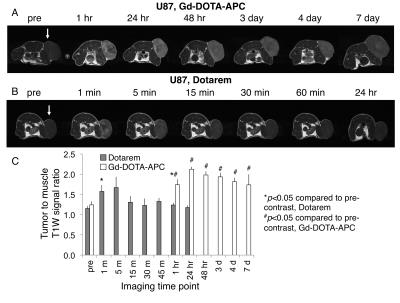
Relaxivity of Gd-DOTA-APC was characterized at 1.5T and 3.0T (GE Signa HDxt and Signa PET/MR, GE Healthcare, Waukesha, WI) at 37°C. Gd-DOTA-APC samples were prepared at concentrations of 0.125-1 mM in water and human plasma. Samples were held at approximately 37°C with a custom-made MRI compatible sample holder and warm water circulation system (Figure 1). For T1 measurement, an inversion recovery pulse sequence with TI=50-750ms, TR=4000ms/5000ms (1.5T/3.0T), and TE=8-9ms was utilized. For T2 measurement, a CPMG sequence with TR=5000ms and TE=25-400ms was utilized. T1 and T2 times were estimated using nonlinear least squares fitting of the signal magnitude vs. inversion time and echo time, respectively. Longitudinal (r1) and transverse (r2) relaxivities of Gd-DO3A-404 were estimated from the slope of the linear relationship between relaxation rate and agent concentration. To examine the stability of Gd3+ in the Gd-DOTA-APC complex, Chelex solid phase extraction was used to separate free Gd3+ from Gd-DOTA-APC dissolved in buffer and measured by magnetic-sector ICPMS after microwave digestion2. Uptake of Gd-DOTA-APC in tumors was investigated in a flank xenograft glioma model (U87) and compared with uptake of Dotarem. Mice (N=3 per group) were delivered approximately 0.12mg/kg contrast intravenously. In vivo imaging was performed on a 4.7T preclinical scanner (Agilent Technologies, Santa Clara, CA) pre-contrast and at multiple time points up to seven days following contrast delivery. R1 maps were estimated using 3D SPGR acquisitions with variable flip angles and B1 field correction3. To assess tumor-specific uptake, the tumor to muscle T1-weighted signal ratio was computed across multiple time points, as shown in Figure 4.Results
At 1.5T, r1 was found to be 16.5 s-1/mM in water and 14.7 s-1/mM in plasma. At 3.0T, r1 was found to be 10.6 s-1/mM in water and 8.45 s-1/mM in plasma (Figure 2). The high longitudinal relaxivity of this agent compares favorably to conventional agents with r1 values ranging from 3.5-7.0 s-1/mM at 1.5T and 2.5-6.5 s-1/mM at 3.0T4. Transverse relaxivities (r2) in water/plasma were 23.9/25.7 s-1/mM at 1.5T and 20.0/25.0 s-1/mM at 3.0T (Figure 3). The initial free fraction of Gd3+ in buffer at room temperature was found to be 0.081%, suggesting high stability consistent with other macrocyclic Gd3+ agents5. After intravenous delivery of Gd-DOTA-APC in mice, specific, sustained uptake was observed in flank U87 xenografts (Figure 4). The tumor to muscle R1 ratio increased 83% (from 0.74 to 1.36) at 48 hours following Gd-DOTA-APC delivery compared to pre-contrast. Significant uptake of Gd-DOTA-APC was sustained from one hour to seven days following administration. In comparison, following intravenous administration of Dotarem, the observed T1-weighted signal enhancement only reached 60% of that resulting from Gd-DOTA-APC, and significant enhancement was not observed beyond one hour post-contrast (Figure 4).Discussion
The results of this study indicate that Gd-DOTA-APC has striking longitudinal relaxivity and high chemical stability, providing evidence of effectiveness and safety for in vivo imaging imaging. In addition, significant tumor uptake and sustained retention of Gd-DOTA-APC was observed in an animal model. These studies indicate that Gd-DOTA-APC holds great potential as a cancer-targeted imaging agent. This approach may also be extended to multi-modality imaging or therapeutics, using DOTA to chelate radiometals or therapeutic radioisotopes.Acknowledgements
No acknowledgement found.References
1. Weichert JP, Clark PA, Kandela IK, et al. Alkylphosphocholine analogs for broad-spectrum cancer imaging and therapy. Sci Trans Med. 2014;6(240):240ra75.
2. Raju CSK, Luck D, Scharf H, et al. A novel solid phase extraction methods for pre-concentration of gadolinium and gadolinium based MRI contrast agents from the environment. J Anal At Spectrom. 2010;25:1573-1580.
3. Yarnykh V. Actual flip-angle imaging in the pulsed steady state: a method for rapid three-dimensional mapping of the transmitted radiofrequency field. Magn Reson Med. 2007;57:192-200.
4. Rohrer M, Bauer H, Mintrovitch J, et al. Comparison of magnetic properties of MRI contrast media solutions at different field strengths. Invest Radiol. 2005;40(11):715-724.
5. Frenzel T, Lengsfeld P, Schirmer H, et al. Stability of gadolinium-based magnetic resonance imaging contrast agents in human serum at 37°C. Invest Radiol. 2008;43(12):817-828.
Figures