0566
A Unique ´Cargo Internalization Receptor (CIR)´ System for In Vivo Tracking of Individual Cell Populations by 19F MRI1Molecular Cardiology, Heinrich-Heine University, Düsseldorf, Germany, 2Biochemistry and Molecular Biology II, Heinrich-Heine University, Germany, 3Pharmaceutical Technology and Biopharmacy, Albert-Ludwigs-University
Synopsis
Synopsis: We demonstrate the capability of unique ‘cargo internalization receptors’ (CIR) for specific cell tracking. A nanobody for green fluorescent protein (GFP) was used to engineer surface receptors which undergo rapid internalization after GFP binding. For 19F MR visibility, the GFP carrier was equipped with perfluorocarbon (PFC) contrast cargo. PFC cargo uptake after CIR transfection was verified by flow cytometry, confocal microscopy, and 1H/19F MRI. The results show that this approach can be successfully used for targeted contrast agent internalization, which can be extended to an cell-specific CIR expression in transgenic mice for in vivo cell tracking by 19F MRI.
Background
Over the last decade, non-invasive cell tracking by 19F MRI has garnered significant interest due to the unique properties of fluorinated materials and the 19F nucleus. 19F is nearly absent from biological tissue, therefore accumulation of 19F-loaded cells results in unequivocal ‘hot spots’ without any background. Furthermore, there are biochemically inert perfluorcarbon emulsions (PFCs) available, which carry a high fluorine payload and can be equipped with specific targeting ligands[1]. However, directing of PFCs to individual surface epitopes remains difficult, since many of the endogenous receptors are not perfectly cell-specific and/or ligand binding induces undesired signalling cascades. Moreover, some surface receptors are not internalized upon binding and therefore the ligand-coupled PFC (cargo) is prone to detach over time from the target cell type. Therefore, we aimed at generating highly specific cargo internalization receptors (CIR) (i) which rapidly internalize targeted PFCs and (ii) whose expression can be tuned in vivo to distinct cell types.Concept
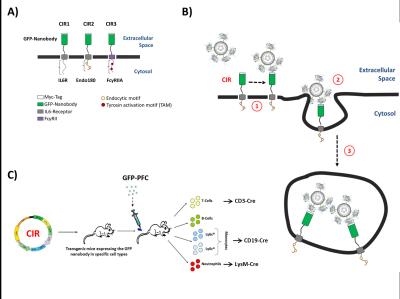
To this end, a nanobody for green fluorescent protein (GFP) was used to engineer surface receptors which undergo rapid internalization after GFP binding (Fig. 1A+B). For 19F MR visibility, the GFP carrier was equipped with contrast cargo, in that GFP was coupled to PFCs (Fig. 2A). To explore the suitability of different uptake mechanisms for this approach, CIRs were constructed by combination of the GFP-specific nanobody and three different cytoplasmic tails that contain individual internalization motifs to enable the endocytosis of the contrast cargo (CIR1, CIR2, and CIR3; Fig. 1A+B). In a final step, the optimized CIR constructs will be cloned (LoxP-flanked) into the Rosa-locus of mice (® CIRfl/fl) to be crossed with strains expressing cre-recombinase under tissue-specific promoters (e.g. LysM-cre, CD19-cre, CD3-cre). This will enable the specific targeting of myeloid cells like neutrophils (LysM-cre) or monocyte subsets but also even non-phagocytic T-cells via GFP-PFCs (Fig. 1C) and may help to unravel the role of distinct immune cell subtypes in a non-invasive manner by 19F MRI.Methods
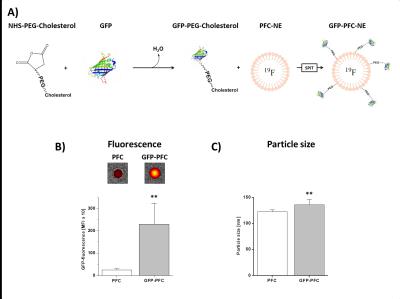
GFP-PFCs were generated using sterol-based post-insertion (SPIT)[2,3]. For this, GFP was linked to cholesterol-PEG1000-NHS (N-hydroxysuccinimide ester) and incubated with preformed PFCs (20% of perfluoro-15-crown-5 ether) that leads to the spontaneous insertion of the cholesterol-moiety into the lipid layer of the PFC (Fig. 2A). CHO cells were transiently transfected with CIR1, CIR2, or CIR3 by lipofection (6 µg plasmid, ~1x106 cells). Stable CIR cell lines were generated by lipofection, followed by geneticin-treatment for several weeks. In the end, stable cell clones were picked and cultivated. For analysis of GFP-PFC uptake, CIR-expressing cells were incubated with GFP-PFCs at 4 °C for 30 min, washed with PBS and further cultivated in medium for 2h at 37 °C to enable internalization of the GFP-PFCs. Finally, the cells were PFA-fixed, pelleted and analyzed by 1H/19F MRI at 9.4T. The tubes were placed in a 25-mm 1H/19F birdcage resonator and analyzed using standard 1H FLASH and 19F RARE sequences. Furthermore, GFP uptake into cells was verified by flow cytometry and confocal microscopy.Results
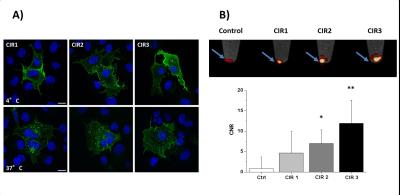
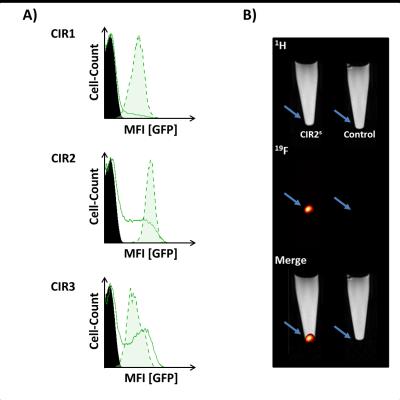
Successful incorporating of GFP-PEG1000-cholesterol into preformed PFCs was assessed by measurement of the fluorescence intensity of PFC/GFP-PFC after purification by ficoll-gradient centrifugation (Fig. 2B; PFC: 253±49; GFP-PFC: 2302±937). As expected, the coupling was associated with a small, but significant increase in size (Fig. 2C; PFCs: 123±3.1 nm; GFP-PFCs: 136±9.9 nm). To verify the specific internalization of GFP-PFC, transiently CIR-transfected cells were incubated with GFP-PFCs at 4 °C - which completely blocks internalization - and at 37 °C enabling the energy-dependent endocytosis of GFP-PFC. While at 4 °C, merely a homogenous fluorescence signal over the cell-surface was observed, incubation at 37 °C resulted in distinct vesicular patterns for all three CIRs, demonstrating the uptake of GFP-PFC into cellular endosomes (Fig. 3A). 1H/19F MRI confirmed the uptake of the targeted emulsion in that increased 19F signals were found in all CIR expressing cells (CNR ctrl: 0.85±2.8; CIR1: 4.65±5.2; CIR2: 6.98±3.3; CIR3: 11.92±5.6) with the strongest signals after CIR2/3-transfection indicating the associated internalization mechanisms to be most efficient for GFP-PFC. The efficacy and sensitivity of the entire approach could be strongly enhanced by generation of stable CIR-expressing cell lines which mimick the conditions in the transgenic target mice. Flow cytometry revealed a much more homogenous and intense uptake of PFC-GFP under stable CIR expression (Fig. 4A) and also initial 19F experiments indicate a clearly increased sensitivity and specificity as compared to control (Fig. 4B).Conclusions
Our results demonstrate that the present approach holds the potential to specifically track the fate of individual cell populations in transgenic CIR-expressing mice which are currently generated.Acknowledgements
This work was supported by the Deutsche Forschungsgemeinschaft (DFG), subproject B2 of the Sonderforschungsbereich 1116.References
1. Kubala MH, Kovtun O, Alexandrov K, Collins BM. Structural and thermodynamic analysis of the GFP:GFP-nanobody complex. Protein Sci. 2010 Dec;19(12):2389-401.
2. Gantert M, Lewrick F, Adrian JE, Rössler J, Steenpass T, Schubert R, Peschka-Süss R. Receptor-specific targeting with liposomes in vitro based on sterol-PEG(1300) anchors. Pharm Res. 2009 Mar;26(3):529-38.
3. Temme S, Grapentin C, Quast C, Jacoby C, Grandoch M, Ding Z, Owenier C, Mayenfels F, Fischer JW, Schubert R, Schrader J, Flögel U. Noninvasive Imaging of Early Venous Thrombosis by 19F Magnetic Resonance Imaging With Targeted Perfluorocarbon Nanoemulsions. Circulation. 2015 Apr 21;131(16):1405-14.
Figures