0559
Detection of Bacteria-specific metabolism using hyperpolarized 13C pyruvate1Radiology and Biomedical Imaging, University of California San Francisco, San Francisco, CA, United States, 2Department of Infectious Diseases, University of California San Francisco, San Francisco, CA, United States
Synopsis
Bacterial infection is a major health problem, with high morbidity and mortality. Current imaging techniques have limited ability to differentiate infection from either tumor or sterile inflammation, and invasive tissue sampling is frequently required. There is currently no clinically-available non-invasive method to directly detect living bacteria in vivo. We describe a method for detecting bacteria specific metabolism using hyperpolarized 13C pyruvic acid.
Aim
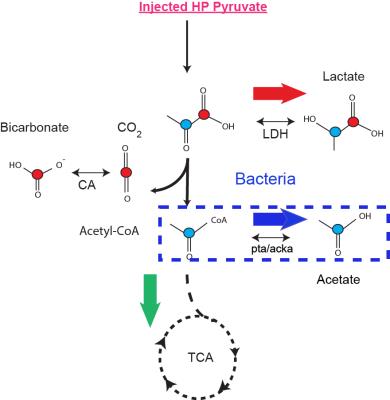
Bacterial infection is a major health problem, with high morbidity and mortality. Current imaging techniques have limited ability to differentiate infection from either tumor or sterile inflammation, and invasive tissue sampling is frequently required. There is currently no clinically-available non-invasive method to directly detect living bacteria in vivo. We describe a method for detecting bacterial metabolism using hyperpolarized 13C pyruvic acid. Because bacteria are much smaller than mammalian cells and they lack organelles, hyperpolarized 13C pyruvate is potentially metabolized rapidly into downstream metabolic products that are not detected in mammalian cells (Figure 1). Specifically, Figure 1 shows the schematic of pyruvate conversion to acetate with the red and blue circles representative of the C1 and C2 carbons of pyruvic acid respectively. The schematic outlines the underlying hypothesis of using C2 pyruvate for detecting bacteria specific metabolism. In normal cells, the energy efficient pathway of Krebs cycle is active, while in cancer cells and activated macrophages (first line of immune response), the glycolytic pathway is dominant. In most bacteria, fermentative production of acetate is the predominant metabolic pathway. Our goal is to determine if production of hyperpolarized acetate could be used to specifically image bacterial infection .Methods:
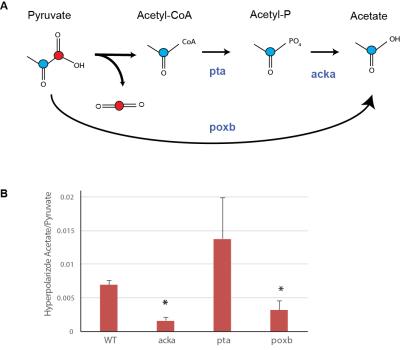
Cultures of E.Coli and S.Aureus were inoculated in 20 ml lysogeny broth (LB) from agar plates and were grown overnight at 37 °C. Shake flask cultures were inoculated from these precultures and grown to the mid-exponential phase (OD600 = 1). Initial MR studies were performed in centrifuged cell pellets resuspended in 500ml of 40 mM HEPES buffer at pH 7.3. For comparative metabolic studies, a renal cell carcinoma cell line (UOK262) and activated macrophages (using lipopolysaccharide, LPS) were studied in MR compatible cell culture bioreactors1. We also tested E.Coli mutants that lacked either pta, acka, and poxb, which comprise key enzymatic steps in projarytoic production of acetate (figure 4). These mutants were compared to wild type after alginate encapsulation in a 5mm bioreactor1 using hyperpolarized [2-13C]pyruvate. 16 µmols each of [1-13C]pyruvate and [2-13C]pyruvate were singly or co-polarized using dynamic nuclear polarization using a 3T Hypersense (Oxford Instruments) and neutralized in a phosphate buffer. The dissolution buffer was added to the cells for dynamic 13C MR measurement in the 11.7T Varian INOVA using 30° pulses, and 3s interval for 300s.Results:
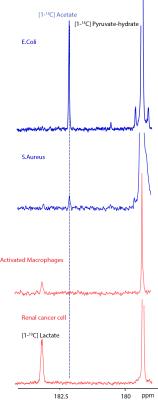
Figure 2 shows the kinetic production of acetate in E.Coli following injection of hyperpolarized [2-13C] pyruvate. The comparative metabolism is shown in figure 3 (the summed spectra over the first 80 seconds), where measureable acetate production was observed in both E.Coli and S.Aureus (representative gram positive bacteria). In contrast, UOK262 renal cell carcinoma and the activated macrophages (bottom spectrum) did not demonstrate any acetate production, but [1-13C]lactate was observed as expected. Additionally, figure 4 shows the two major pathways of acetate production from pyruvate via the poxb and pta/acka enzyme systems. As expected, in bacteria that lack either acka or poxb, the hyperpolarized acetate/pyruvate signal is significantly decreased by 28±7% and 45±20% respectively (p<0.05), compared to the wildtype E.Coli. In addition, the acka mutant shows buildup of intermediates such as acetyl-coA. Finally, E.Coli that lack pta can still make acetate, suggesting a dominant role for the POX – PTA/ACK pathway. The bar graph (figure 4) demonstrates persistent acetate production in the absence of each of the aforementioned enzymes, clearly indicating the central role of acetate in bacterial metabolism.Discussion and Conclusion:
Acetate plays a key role as a metabolic switch in bacterial growth and survival. In this work, we have demonstrated that hyperpolarized acetate production from pyruvate in pathologic bacteria is observable and distinct from the signals observed in mammalian cells. Additionally, we have demonstrated the persistent hyperpolarized acetate production in multiple E.coli variants lacking key enzymes, reaffirming the central role of acetate in bacterial metabolism. Further experiments are underway to gauge the sensitivity of this technique for in vivo translation. However, these preliminary results suggest that production of hyperpolarized acetate can serve as a biomarker of bacterial infection.Acknowledgements
We would like to acknowledge Sukumar Subramaniam, Dave Korenchan, Tiffany Kwak, Robert Bok and Romelyn DeLos Santos for their help with experimentsReferences
1. Keshari KR, Wilson DM, Van Criekinge M, Sriram R, Koelsch BL, Wang ZJ, VanBrocklin HF, Peehl DM, O'Brien T, Sampath D, Carano RA, Kurhanewicz J. “Metabolic response of prostate cancer to nicotinamide phophoribosyltransferase inhibition in a hyperpolarized MR/PET compatible bioreactor. Prostate.” 2015 Oct;75(14):1601-9. doi: 10.1002/pros.23036.
2. Wolfe AJ. “The acetate switch.” Microbiol Mol Biol Rev. 2005 Mar;69(1):12-50.
3. Rae C, Fekete AD, Kashem MA, Nasrallah FA, Bröer S. “Metabolism, compartmentation, transport and production of acetate in the cortical brain tissue slice”. Neurochem. Res. 2012 Nov;37(11):2541–53.
4. Choudhary C, Weinert BT, Nishida Y, Verdin E, Mann M. The growing landscape of lysine acetylation links metabolism and cell signalling. Nat. Rev. Mol. Cell Biol. 2014 Aug;15(8):536–50. 17.
5. Hatzivassiliou G, Zhao F, Bauer DE, Andreadis C, Shaw AN, Dhanak D, Hingorani SR, Tuveson DA, Thompson CB. ATP citrate lyase inhibition can suppress tumor cell growth. Cancer Cell. 2005 Oct;8(4):311–21.
Figures