0558
Toward Dynamic 3D Cardiac Perfusion Imaging Using bSSFP and Hyperpolarized tert-Butanol1Radiology, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA, United States
Synopsis
Perfusion imaging is a promising application for hyperpolarized tracers, as they provide high signal with no endogenous background. Hyperpolarized 13C labeled tert-butanol is a perfusion agent with long T1 and T2 relaxation times in vivo. Moreover, because it diffuses freely through tissue, bolus injections of tert-butanol are largely extracted from the vasculature on a first pass, and the residence time in tissue is on the order of tens of seconds. This provides a long time frame for dynamic imaging. Here we demonstrate the feasibility of time-resolved 3D cardiac perfusion imaging in rats.
Purpose
To investigate the use of balanced steady-state free precession (bSSFP) for 3D hyperpolarized cardiac perfusion imaging.Introduction
Hyperpolarized MRI holds promise for cardiac perfusion imaging, as it enables imaging at high spatial resolution with good sensitivity and no endogenous background. Previous studies [1] have demonstrated use of a prospectively gated spiral acquisition to image hyperpolarized urea in the heart. Here we describe an alternative approach using hyperpolarized tert-butanol [2], a tracer that can diffuse freely through tissue. Owing to this diffusibility, bolus injections of tert-butanol are largely extracted from the vasculature on a first pass, resulting in a reduction of the blood pool signal. Furthermore, the large distribution volume of the tracer implies a long residence time in tissue, on the order of tens of seconds. These features enable use of 3D imaging sequences with retrospective cardiac gating.Materials and Methods
Imaging was performed using a 9.4T horizontal bore scanner (Biospec 94/20, Bruker, Billerica MA) equipped with a 1H 84mm quadrature volume coil and a 28mm transmit/receive surface 13C coil. Proton cardiac cine images were acquired using a self-gated FLASH sequence (Bruker IntraGate). For 13C imaging, a standard 3D bSSFP sequence was modified by elimination of the slice select gradients and the introduction of an adiabatic tip angle ramp to gradually increase the tip angle from zero at the start of each 3D frame and return it to zero at the end of each frame. Data synchronization was achieved by programming the scanner to switch a TTL signal at the beginning of each readout. In vivo experiments were performed with IACUC approval. Fischer rats, weight approximately 250g, were anesthetized using isoflurane in oxygen. After placement of a tail vein catheter, animals were situated in a prone position inside the magnet with respiratory and temperature monitoring, and thermal support provided by a warm air circulator. The 13C surface coil was placed directly under the heart. ECG monitoring was performed using a MR-compatible gating system (Model 1030, SAII, Stony Brook NY). The scanner TTL signal and the output of the cardiac gating system were recorded using a data acquisition board connected to a PC. Hyperpolarized tert-butanol was prepared using a DNP hyperpolarizer (Hypersense, Oxford Instruments, Oxfordshire UK) as described previously [2]. Following acquisition of proton reference images, a 3D 13C bSSFP scan was prescribed with TR=2ms, 32x32x40 matrix size, 1.5mm isotropic resolution, and tip angle approximately 5º. In some acquisitions, to reduce the 13C signal in the blood pool, saturation bands were placed superior and inferior (cranial and caudal) to the heart to null the signal in venous blood returning to the heart. 13C image acquisition was initiated 10s after completing a 2.5ml injection of 250mM tert-butanol. 32 consecutive frames were acquired over 60 seconds. Image reconstruction was performed in two steps. First, images were reconstructed using Fourier transforms without retrospective gating, and the rate constant of the magnetization decay was determined by fitting the signal in the heart to an exponential e-Rt. Next, the first 16s of data were rescaled by a factor e+Rt to correct for magnetization decay, and then sorted into 6 bins based upon acquisition time within the cardiac cycle [3]. These 6 data sets were then reconstructed to form cine perfusion-weighted images. Lastly, static images at diastole were obtained by reconstructing the average of 3 bins that excluded systole.Results and Discussion
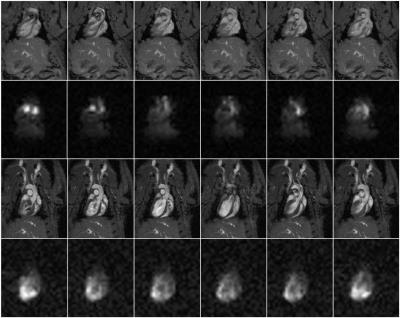
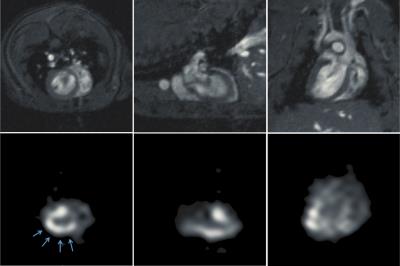
In a representative study making use of saturation bands, an exponential fit to the magnetization decay yielded a rate constant R-1=20.6±0.5s, indicating that tert-butanol has a relatively long residence time in tissue. Fig. 1 shows representative proton and 13C cine images acquired without (top two rows) and with (bottom two rows) saturation bands placed superior and inferior to the heart. In the absence of saturation bands, the images show bright signal in the blood pool that partially obscures the myocardium. This effect is markedly reduced in the lower two rows. Fig. 2 shows proton and perfusion-weighted 13C images at end diastole in axial, coronal and sagittal planes extracted from a single 3D volume. Although some residual blood signal remains, strong perfusion signal is visible in the myocardium, particularly in areas adjacent to the 13C surface coil. The SNR within the myocardium in the region of Fig. 2 indicated by the arrows ranges from 22 to 28.Conclusions
These data show that tert-butanol has a long persistence time in the myocardium, which can be exploited for 3D retrospectively gated cardiac perfusion imaging in rats. Bright blood signal can be reduced by introduction of saturation bands, improving visualization of the myocardium.Acknowledgements
This work was supported in part by the NIH through grants R21 EB014471 and R01 CA169470References
[1] Lau AZ, Miller JJ, Robson MD, and Tyler DJ. Cardiac Perfusion Imaging Using Hyperpolarized 13C Urea Using Flow Sensitizing Gradients. Magn Reson Med 75:1474-1483 (2016).
[2] Grant AK, Vinogradov E, Lenkinski RE, Alsop DC. Perfusion Imaging with a Freely Diffusible Hyperpolarized Contrast Agent. Magn Reson Med 66:746-755 (2011).
[3] Miraux S, Calmettes G, Massot P, Lefrancois W, Parzy E, Muller B, Arsac LM, Deschodt-Arsac V, Franconi JM, Diolez P, Thiaudiere E. 4D Retrospective Black Blood TrueFISP Imaging of the Mouse Heart. Magn Reson Med 62:1099-1105 (2009).