0557
In vivo metabolic imaging of neuroinflammation following traumatic brain injury using hyperpolarized [1-13C] pyruvate1Brain and Spinal Injury Center, University of California San Francisco, San Francisco, CA, United States, 2Department of Physical Therapy and Rehabilitation Science, University of California San Francisco, San Francisco, CA, United States, 3Department of Neurological Surgery, University of California San Francisco, San Francisco, CA, United States, 4Surbeck Laboratory of Advanced Imaging, Department of Radiology and Biomedical Imaging, University of California San Francisco, San Francisco, CA, United States
Synopsis
This study demonstrates that metabolic imaging of hyperpolarized [1-13C] pyruvate can detect increased hyperpolarized lactate production in vivo in a preclinical model of Traumatic Brain Injury. Correlative assays further demonstrate that this increased lactate is linked to the presence of pro-inflammatory macrophages that upregulate pyruvate dehydrogenase kinase 1, subsequently leading to regional pyruvate dehydrogenase inhibition. Metabolic imaging of hyperpolarized [1-13C] pyruvate thus has great potential to provide a novel tool for in vivo detection of neuroinflammation.
INTRODUCTION
Traumatic brain injury (TBI) induces chronic activation of mononuclear phagocytes (microglia/macrophages, MPs), which persists for many years following initial insult. Recent studies have shown that modifying early MPs response can rescue long term cognitive deficits, highlighting the critical and early role of pro-inflammatory MPs in TBI progression1. Importantly, novel studies have shown that the activation status of MPs is associated with a distinct metabolic reprogramming: proinflammatory MPs increase glycolysis and lactate release, whereas anti-inflammatory MPs rely on oxidative pathways2,3. In this context, we questioned whether hyperpolarized 13C magnetic resonance spectroscopic imaging (HP 13C MRSI) of pyruvate could be used to monitor MPs status in vivo. We demonstrated that HP 13C lactate/pyruvate ratios were increased in the injured cortex at acute (12 and 24 hours) and sub-acute (7 days) time points. We further showed that increased HP [1-13C] lactate/pyruvate ratios were associated with the presence of pro-inflammatory MPs expressing pyruvate dehydrogenase kinase 1 (PDK1) and a decreased activity of pyruvate dehydrogenase (PDH) activity in the injured cortex, providing a likely mechanism for the increased flux towards lactate production.METHODS
Animals: C57BL/6J mice (n=8) received TBI using the controlled cortical impact model (CCI)1. Mice received a ~3.5 mm diameter craniectomy at -2.0 mm (anteroposterior)/ +2.0 mm (mediolateral) with respect to bregma, leading to a contusion depth of 0.95 mm (from dura). Velocity was constant at 4 m/s, and the impact was sustained for 300 ms.
MR acquisitions: Mice were imaged prior (Baseline) and at 12/24 hours, 7/28 days post-injury. T2-weighted images were acquired for assessment of injury location and volume (TE/TR=20/1200ms, thickness=0.5mm, NA=2, matrix=256x256, FOV=30x30mm²). For 13C MRS, 24μL of [1-13C] pyruvate preparation was hyperpolarized using a Hypersense DNP polarizer (Oxford Instruments) for one hour4. After dissolution, HP pyruvate was rapidly dissolved in isotonic buffer (pH~7) and injected intravenously (iv) over 12sec. From the beginning of iv injection, 2D dynamic CSI 13C data were acquired on a 14.1T MR system using: TE/TR=1.2/60ms; SW=2500Hz; 128points; 4sec resolution; FA=10deg; FOV=24x24 mm²; 5mm thickness.
Immunofluorescence (IF): IF analyses were performed for MPs (Iba-1), astrocytes (GFAP), PDK1 and nuclei (Hoechst). PDH was assessed using a PDH activity assay kit (Abcam).
Data analysis: The volume of the lesion, cavity and ventricles was determined after delineation on T2-weighted images and compared to Baseline values using a repeated measure statistical test. HP [1-13C] lactate and pyruvate levels were calculated as the sum of integrals. HP [1-13C] lactate/pyruvate ratios evolution over time was evaluated using a two-way anova. PDH enzyme activity assay was evaluated using a paired t-test (*p<0.05, **p<0.01, ***p<0.005).
RESULTS
Anatomical alterations were detected on high resolution T2-weigthed MRI. The presence of TBI lesions was observed as early as 12h post-injury and the formation of a cavitation at 7 and 28 days post-injury (Fig.1).
Next, HP 13C MRSI data were collected to evaluate metabolic alterations following injury. Dynamic acquisition of HP 13C spectra from the TBI voxel (red) showed the increase of HP 13C lactate as compared to the contralateral voxel (blue) at 7 days post-injury (Fig.2A). The corresponding HP [1-13C] lactate/pyruvate ratio heatmap indicated that this increase was restricted to the injury site (Fig.2B). When comparing the TBI (red) with the contralateral (blue) voxel (Fig.2C), we found that the HP-13C lactate/pyruvate ratios, expressed as percentage change compared to baseline levels, are significantly increased in the TBI voxel at 12h (p=0.0005), 24h (p=0.0002) and 7 days (p<0.0001).
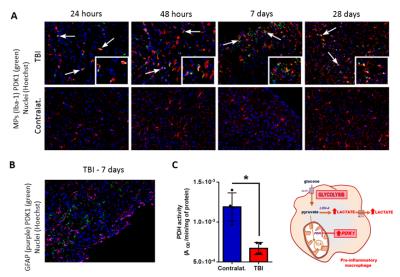
Importantly, IF analyses showed that PDK1 expression (green) could only be detected in the TBI region where it co-localized with round/amoeboid-shaped activated MPs at 24 and 48 hours, and 7 and 32 days post-injury (Fig.3A, white arrows, inserts). Furthermore, PDK1 was not detected in astrocytes at any time points post-injury (Fig.3B). In line with the increased PDK1 expression, PDH activity was decreased in the injured cortex as compared to the contralateral cortex at 7 days post-injury (p=0.0116) (Fig.3C). Further analyses are currently on going to evaluate PDH activity at each time point.
CONCLUSIONS
In conclusion, our results
demonstrate that 13C MRSI of [1-13C] pyruvate can detect increased lactate
production in vivo in a preclinical model
of TBI, and that this increase is linked to the presence of activated MPs.
Importantly, this report is the first to demonstrate the use of a metabolic
imaging method to monitor TBI-induced neuroinflammation. Because HP 13C
MRSI is expanding rapidly, this study is of high significance for future
clinical trials not only on TBI, but also all neurological diseases presenting
inflammatory components.Acknowledgements
NMSS_PP3395; Cal-BRAIN349087; UCSF_RAP7500634; UCSF Department of Radiology seed grants #14-04 & #14-05; NIH-funded Hyperpolarized MRI Technology Resource Center #P41EB013598; NIH/NINDS R21AG042016, NS087458 and R21NS096718.References
[1] Morganti et al., CCR2 Antagonism Alters Brain Macrophage Polarization and Ameliorates Cognitive Dysfunction Induced by Traumatic Brain Injury, J. of Neuroscience (2015) 35(2): 748-760
[2] Galvan-Pena et al, Metabolic Reprograming in Macrophage Polarization, Front. Immunol.(2014) Sep 2;5:420.
[3] Tan et al, Pyruvate dehydrogenase kinase 1 participates in macrophage polarization via regulating glucose metabolism J. Immunol. (2015) Jun 15;194(12):6082-9
[4] Kurhanewicz et al, Analysis of cancer metabolism by imaging hyperpolarized nuclei: prospects for translation to clinical research, Neoplasia (2011) Feb;13(2):81-97.
Figures