0556
Hyperpolarized 13C MRS of the Human HeartAlbert P. Chen1, Justin Y.C. Lau2,3, Benjamin J. Geraghty2,3, William J. Perks4, Idan Roifman5, Graham A. Wright2,3,5, Kim A. Connelly6, and Charles H. Cunningham2,3
1GE Healthcare, Toronto, ON, Canada, 2Physical Sciences, Sunnybrook Research Institute, Toronto, ON, Canada, 3Medical Biophysics, University of Toronto, Toronto, ON, Canada, 4Pharmacy, Sunnybrook Health Sciences Centre, Toronto, ON, Canada, 5Schulich Heart Program, Sunnybrook Health Sciences Centre, Toronto, ON, Canada, 6Cardiology, St. Michael’s Hospital, Toronto, ON, Canada
Synopsis
The feasibility of acquiring hyperpolarized 13C images from human hearts following injections of HP [1-13C]pyruvate solution has been recently demonstrated. Following the rapid multi-slice 13C imaging acquisition, 13C spectroscopic data were acquired from the whole heart to detect any residual hyperpolarized magnetization. 13C pyruvate, lactate, alanine and bicarbonate were observed in all 5 subjects. 13CO2 was also detected in two of the datasets. Good inter-subject consistency in the observed metabolite ratios from these spectra demonstrated the potential for changes in cardiac metabolism due to disease to be interrogated with simple whole-heart spectroscopy.
Introduction
Hyperpolarized 13C MR imaging and spectroscopy have been used to investigate changes in metabolism in the heart related to various diseases and physiological perturbations in pre-clinical models and has shown tremendous clinical potential1. The feasibility of acquiring hyperpolarized 13C images from human hearts following injections of HP [1-13C]pyruvate solution has been recently demonstrated2. Following the rapid multi-slice 13C imaging data acquisition, 13C spectroscopic data were acquired from the whole heart to detect any residual hyperpolarized magnetization. The spectroscopic data obtained from this add-on acquisition provided complementary information that helps to interpret the imaging results and to design acquisition methods in future studies.Methods
Subjects: Healthy subjects (N=5) were recruited and gave written informed consent under a protocol approved by the institutional Research Ethics Board and approved by Health Canada as a Clinical Trial Application. An oral carbohydrate load (35g of GatoradeTM powder in water, containing 34g of sucrose and dextrose) was administered approximately 1 hour before the pyruvate injection. Hardware and sample preparation: Each subject was positioned supine and feet first within a 13C volume transmit coil system (GE Healthcare) installed on a GE MR750 3.0 Tesla MRI scanner (GE Healthcare). The 13C receiver coil system consisted of two separate paddles each containing four receiver elements. One paddle was positioned on the anterior chest wall over the heart, with the other paddle under the upper left back. Hyperpolarized [1-13C] pyruvate solution was prepared using a GE SPINLabTM system equipped with the Quality Control (QC) module. After sample dissolution, the QC parameters were evaluated by the study pharmacist to ensure the parameters were within specifications. Upon release, the dose syringe was rapidly loaded onto a Spectris Solaris power injector (Medrad) and a 0.43 cc/kg dose of the ~250mM pyruvate solution was injected at 5mL/s followed by a 25mL saline flush at 5mL/s. 13C MRS acquisitions: The 13C imaging data acquisition was initiated at the end of the saline flush and required 18 cardiac cycles to complete (14 - 21s depending on heart rate). Following the 13C imaging2,3, the residual hyperpolarized magnetization was used to acquire MR spectroscopic data from the whole heart. For the first subject, a slice-selective pulse was used (nominal flip angle = 30°, 10 cm axial slab covering the heart) and the acquisition was not cardiac gated (TR = 3s). For the four subsequent subjects, a 200µs non-selective RF pulse was used (nominal flip angle = 18°) and the acquisition was cardiac gated such that the TRs were approximately 3s (3 to 4 inter-beat intervals, depending on heart rate). The shorter RF pulse was intended to excite the 13CO2 resonance along with the other metabolites.Results and Discussion
For the 5 subjects, the average starting time of the 13C MRS acquisition was 56s after the start of hyperpolarized 13C pyruvate injection. Despite the long delay time and the consumption of most of the pre-polarized magnetization by the preceding multi-slice 13C imaging acquisition, 13C pyruvate, lactate, alanine and bicarbonate were observed in all subjects. 13CO2 was also detected in two of the datasets acquired using the non-selective pulse from the first 4-5 spectra (Fig. 1) and intracellular pH values estimated from these data sets were 7.1 and 7.3. The lactate and bicarbonate to pyruvate ratios are shown in Fig. 2. For the 4 datasets acquired using the non-selective pulse (subjects 2-5), the metabolite / pyruvate ratios were similar over the time period that the peaks had adequate SNR (> 10). Subject 1 had significantly higher metabolite / pyruvate ratios, likely because the slice selective pulse excited less of the pyruvate in the cardiac chambers compared with the non-selective pulse. The average linewidth from the 5 datasets for 13C pyruvate, lactate, and bicarbonate were 20.1Hz, 25.0Hz, and 19.2Hz, respectively. The broad linewidths were likely due to the data being acquired from the whole heart, and the larger linewidth for 13C lactate may also be associated with the observation from the imaging. The bicarbonate-to-pyruvate ratios remained relatively stable during the time points with sufficient SNR for quantification, ranging from 20 to 70 seconds. In contrast, the lactate-to-pyruvate ratio increased over a similar time frame for all subjects (Fig. 2).Conclusion
13C MRS data acquired from the whole heart in normal human subjects after injection of hyperpolarized [1-13C]pyruvate and multi-slice 13C imaging provided additional information not available in the imaging data. The inter-subject consistency in the observed metabolite ratios from these spectra demonstrated the potential for changes in cardiac metabolism due to disease to be interrogated with simple whole-heart spectroscopy.Acknowledgements
The authors are grateful to Tracey Rideout, Sergio DeFigueiredo and Stephanie Vidotto for pharmacy technician support for this study, to Julie Green for coordinating the study, and to Ruby Endre and Garry Detzler for MR technician support.References
1. Schroeder MA et al. Circ. 2011;124:1580-94. 2. Cunningham CH et al. Circ Res. 2016;Epub. 3. Lau AZ et al. MRM. 2010;64:1323-31.Figures
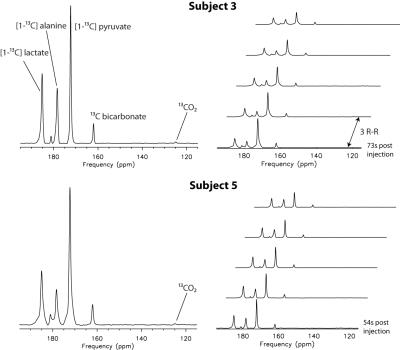
Fig.1. 13C
MRS data from 2 of the 5 subjects with the first 5 spectra from each dataset
shown (summed spectra on the left and individual spectra in stack view on the
right). 13CO2
signals were detected in these studies.
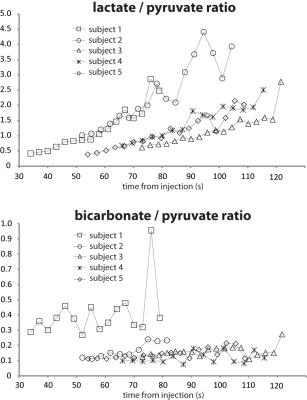
Fig. 2. 13C
lactate to pyruvate ratios and 13C bicarbonate to pyruvate ratios
from the 5 normal subjects. The 4
datasets acquired with the same non-selective RF pulse showed good agreement
with each other.