0549
Cardiac fMRI - A Novel Approach for Reliably Detecting Myocardial Oxygenation Changes with Precise Modulation of Arterial CO21Cedars Sinai Medical Center, Los Angeles, CA, United States, 2IMT School for Advanced Studies Lucca, 3University of Toronto, 4Lawson Health Research Institute, 5Siemens Healthcare
Synopsis
Although cardiac BOLD MRI can detect ischemic heart disease without ionizing radiation and contrast agents, its reliability remains poor due to the low sensitivity and specificity. We propose a novel strategy to overcome these barriers through: (i) an improved MRI strategy with free gas-exchange capability; (ii) repeat stimulation of heart using a validated prospective arterial CO2 targeting technique; and (iii) a statistical framework to increase the confidence measure of BOLD signal changes. Our results show that the proposed approach can be used to significantly increase the confidence in detecting myocardial BOLD response in conditions of health and disease.
Introduction
Cardiac BOLD MRI has evolved into a promising method for detecting ischemic heart disease without ionizing radiation or contrast agents. Nonetheless, in spite the technical advancements over the past 20 years that have overcome major obstacles, its reliability remains poor. This limitation is fundamentally a consequence of low sensitivity and specificity of weak myocardial BOLD image contrast. In this study, we propose a novel strategy to overcome these barriers through: (i) a confounder-corrected 3D T2 mapping at 3T (1) that is performed at high-resolution with whole-heart coverage, rapid data collection, and free-breathing acquisitions permitting free gas-exchange capability; (ii) repeat stimulation of heart using a validated prospective arterial CO2 (PaCO2) targeting technique (2); and (iii) a statistical framework to increase the confidence measure of BOLD signal changes. We demonstrate herein that the proposed approach enables a unique opportunity to reliably capture the BOLD signal changes across multiple measurements during CO2-mediated coronary vasodilation through unprecedented amplification in sensitivity and specificity in health and disease.Methods
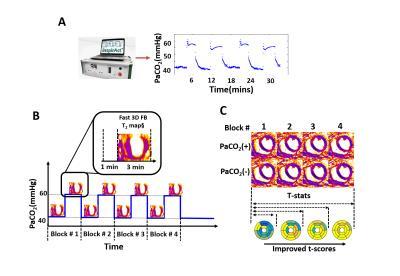
Fast, confounder-corrected, 3D BOLD MRI with precisely targeted PaCO2:
Canines with (n=5) and without (n=5) surgically controlled LAD stenosis were studied in a 3T clinical PET/MR system. A fast, confounder-corrected, free-breathing 3D T2 mapping sequence (as previously described) was prescribed during rest, under adenosine and under repeat arterial CO2 level (PaCO2) modulations (baseline/normocapnic PaCO2 [PaCO2(-): 40mmHg]; hypercapnic PaCO2 [PaCO2(+): 60mmHg]. T2 mapping employed SR-preparation with FLASH readout: TR/TE=3.2/1.6ms, flip angle=15°, spatial resolution=2x2x5mm3 (14 partitions), adiabatic T2-prep pulses and SR recovery time=350ms. Dynamic 13N-ammonia PET scans were acquired for validation at rest and under adenosine. The repeat PaCO2 modulation and acquisition protocol are illustrated in Figs. 1 (A and B). 3D T2 maps were acquired during 4 blocks of repeat PaCO2 modulation (each block consisted 4 minutes normocapnia and 4 minutes of hypercapnia).
Image processing and statistical framework:
To utilize the multiple measurements from repeated PaCO2 modulations, all 3D T2 maps were registered to the initial PaCO2(-) T2 map using an image processing package (ANTs, stnava) to avoid inter-acquisition motion. The registered images were segmented according to the AHA for analysis. Segmental myocardial T2 values acquired under repeat PaCO2(-) and PaCO2(+) were compared using t-statistic to test the null hypothesis:
H0 [Null: BOLD response present]: T2 during PaCO2(-) = T2 during PaCO2(+)
H1 [Alternate: BOLD response absent]: T2 during PaCO2(-) ≠ T2 during PaCO2(+)
Segmental t-scores and confidence of responses (CoR) were derived from integrated myocardial T2 values from repeat PaCO2 modulations (Fig. 1C). t-score was defined as: [meanT2[PaCO2(+)]-meanT2[PaCO2(-)]/standard error[PaCO2(+), PaCO2(-)]); and CoRs were derived using the t-score and the degree of freedom of the measurements. Null hypotheses were rejected if CoR>0.95.
Results
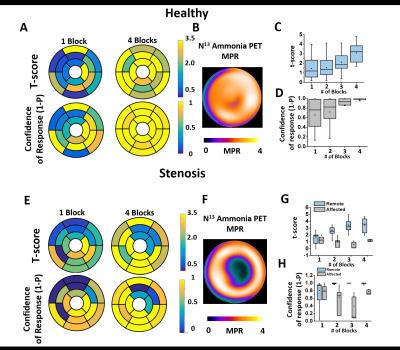
Figure 2 shows representative findings in a healthy animal and an animal with coronary stenosis. Healthy Subjects: Panel A shows representative t-scores and CoR maps derived from T2 maps acquired using 1 block and 4 blocks of PaCO2 stimulation. Note that with increasing number of PaCO2 blocks both the t-scores and CoRs are increased; and that with 4 blocks of PaCO2 modulation, all segments reach CoRs >0.95. Panel B shows that PET myocardial perfusion reserve (MPR) is substantially greater than 2 from the same animal. Panels C and D show quantitative comparisons of t-scores and CoRs. These findings were consistent across all animals. Coronary Stenosis: Panels E, F, G, and H show representative results from an animal with LAD stenosis. Note that while a similar trend to the healthy subjects are evident in the remote segments, the affected segments show no increase in t-scores or CoRs with increased blocks. Panel E shows significantly lower t-scores and CoRs in the LAD territories from maps with 4 blocks while no obvious spatial BOLD response is observable in maps acquired with 1 block. Corresponding PET MPR (panel F) shows obvious perfusion deficit in the affected territories as identified by maps acquired with 4 blocks. Panels G and H show vastly improved capability to differentiate between remote and affected segments with increasing number of blocks. These findings were also consistent across all animals.Conclusions
Our findings here demonstrate that repeat coronary stimulations with precisely targeted changes arterial CO2 can be used to significantly increase the confidence in detecting myocardial BOLD response under conditions of health and coronary stenosis. We expect our early findings to pave the way for reliable the detection and quantification of ischemic myocardial volume without contrast agents or ionizing radiation on the basis of BOLD MRI, and thereby enabling accurate assessment of ischemic heart disease especially in patients with advanced renal dysfunction.Acknowledgements
No acknowledgement found.References
1. Yang HJ, Sharif B, Pang J, Kali A, Bi X, Cokic I, Li D, Dharmakumar R. Free-Breathing, Motion-Corrected, Highly-Efficient Whole-Heart T2 Mapping at 3T with Hybrid Radial-Cartesian Trajectory. Magn Reson Med. 2015 Mar 6. doi: 10.1002/mrm.25576.
2. Yang HJ, Yumul R, Tang R, Cokic I, Klein M, Kali A, Sobczyk O, Sharif B, Tang J, Bi X, Tsaftaris SA, Li D, Conte AH, Fisher JA, Dharmakumar R. Assessment of Myocardial Reactivity to Controlled Hypercapnia with Free-breathing T2-prepared Cardiac Blood-Oxygen-Level-Dependent MR Imaging. Radiology. 2014 Apr 17:132549.
Figures