0548
First-Pass Nitroxide-Enhanced MRI for Imaging Myocardial Perfusion without Gadolinium1Biomedical Engineering, University of Virginia, Charlottesville, VA, United States, 2Radiology, University of Virginia, Charlottesville, VA, United States
Synopsis
First-pass MRI using gadolinium-based contrast agents is widely used to image myocardial perfusion. However, gadolinium is contraindicated for patients with severely impaired renal function (a substantial portion of heart disease patients), and methods that do not employ gadolinium are needed. Nitroxide stable free radicals are non-metallic compounds with an unpaired electron and, correspondingly, are paramagnetic and T1-shortening. We investigated first-pass nitroxide-enhanced perfusion MRI of the heart as an alternative to first-pass gadolinium-enhanced MRI. Five C57Bl/6 mice underwent first-pass imaging with the nitroxide agent 3CP and the results showed that nitroxide-enhanced MRI can quantify regional myocardial blood flow, as the average myocardial perfusion was 7.0±1.3 ml/g/min, a value in the normal range for mice.
Introduction
First-pass MRI using gadolinium-based contrast agents is widely used to image myocardial perfusion. However, gadolinium is contraindicated for patients with severely impaired renal function (a substantial portion of heart disease patients), and methods that do not employ gadolinium are needed. Arterial spin labeling is a potential alternative, but has low perfusion signal-to-noise ratio. Nitroxide stable free radicals are non-metallic compounds with an unpaired electron and, correspondingly, are paramagnetic and T1-shortening. However, nitroxide contrast agents are not commonly used in MRI because they undergo in vivo reduction reactions and lose their T1-shortening property over the course of several minutes. With this property, nitroxides have been used as redox-sensitive MRI contrast agents in preclinical cancer imaging to assess tumor redox status [1, 2]. Since first-pass perfusion imaging occurs on a time scale on the order of seconds, we investigated first-pass nitroxide-enhanced perfusion MRI of the heart as an alternative to first-pass gadolinium-enhanced MRI.Methods
The nitroxide contrast agent 3-Carbamoyl-PROXYL (3CP) (Sigma–Aldrich, St. Louis, MO) was chosen because it is water soluble, commercially available and well tolerated by mice. The relaxivity of 3CP at 7T was measured using a series of 12 phantoms with a range of 3CP concentrations. The phantom’s T1 values were measured using a Look-Locker sequence. Relaxivity was calculated by least square fitting of the following equation: 1/T1 = 1/T1baseline + Γ · [3CP]. Wild-type male C57Bl/6 mice (n = 5) underwent MRI studies using a 7T system (Clinscan, Bruker). The ECG, body temperature, and respiration were monitored during imaging using an MR-compatible system (SA Instruments, Stony Brook, NY). During MRI, mice were anesthetized with 1.25% isoflurane and maintained at 36 ± 0.5°C using circulating warm water. After localizer imaging, first-pass perfusion MRI was performed in a mid-ventricular short-axis slice. 3CP was administered through an indwelling tail vein catheter at 2 mmol/g body weight over 3 to 4 seconds. The concentration in the bolus solution was 50 mg/mL. Rate-3 undersampled saturation-recovery rapid gradient echo imaging was used with: field of view= 34 x 24 mm2, matrix size = 128 x 102, TE/TR = 1/1.8 ms, flip angle = 15°, slice thickness = 1 mm, and saturation delay = 45 ms. Images were reconstructed using BLOSM [3], a compressed sensing method. Signal intensities of blood and myocardial regions were normalized by proton density images and were converted to 3CP concentrations using the methods described by Cernicanu and Axel [4], and perfusion was quantified using Fermi function deconvolution [5]. Regional perfusion was assessed in regions of interest defined as anterior, anterolateral, inferolateral, inferior, inferoseptal, and anteroseptal according to a standard American Heart Association segment model. To compare the signal enhancement from 3CP to that achieved using a standard dose of Gd (0.1 mmol/kg), Gd-enhanced first-pass MRI was also performed and the blood and myocardial peak enhancement ratios were calculated for 3CP and gadolinium using the following formula: percentage enhancement = (SIpost − SIpre) / SIpre × 100%, where SIpre is the pre-contrast signal intensity and SIpost is the peak post-contrast signal intensity.Results
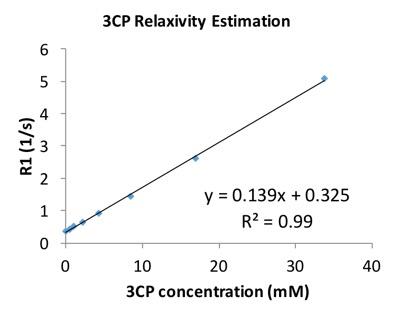
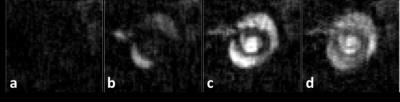
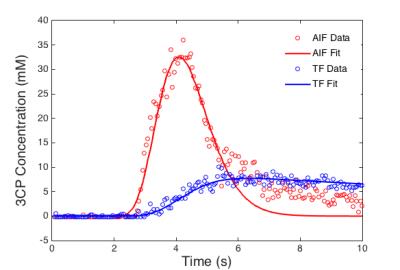
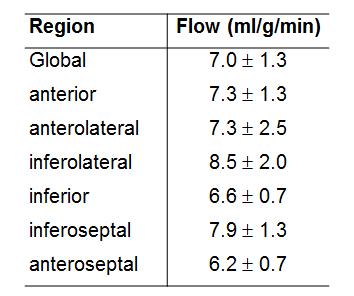
In phantoms, the relaxation rates R1 (or 1/T1) increased linearly with 3CP concentration in the range of 0.5 to 35 mM at 7T (Figure 1), and the relaxivity of 3CP in saline solution was measured to be 0.139 mM-1sec-1. Figure 2 shows example 3CP-enhanced first-pass MR images obtained from a mouse before (a) and after (b,c,d) injection of 3CP, and Figure 3 shows example arterial input function (AIF) and tissue function (TF) data and fits obtained from a mouse. Table 1 summarizes the global and regional myocardial perfusion values (mean ± standard error) from 5 mice using 3CP-enhanced first-pass MRI. The percentage enhancement at peak signal enhancement achieved by 3CP at 2 mmol/g (n = 5) were 725 ± 100% and 275 ± 41% for blood and myocardium, respectively, compared to 1130 ± 60% and 342 ± 21%, respectively, for gadolinium at 0.1 mmol/kg (n = 5).Conclusion and Discussion
We demonstrated that first-pass nitroxide-enhanced MRI, a non-gadolinium technique, can quantify myocardial perfusion in mice. As shown, 3CP generated substantial enhancement of the blood and myocardium, and the perfusion values estimated are within the normal range for mice [6]. While nitroxides have not been tested in humans, in principle nitroxide-enhanced MRI could translate to humans as these compounds may have sufficient safety profiles.Acknowledgements
Funding: NIH R01 EB001763 and AHA pre-doctoral fellowship 16PRE29750008.References
1. Hyodo, F., et al., Probing the intracellular redox status of tumors with magnetic resonance imaging and redox-sensitive contrast agents. Cancer Res, 2006. 66(20): p. 9921-8.
2. Matsumoto, K., et al., High-resolution mapping of tumor redox status by magnetic resonance imaging using nitroxides as redox-sensitive contrast agents. Clin Cancer Res, 2006. 12(8): p. 2455-62.
3. Chen, X., et al., Motion-compensated compressed sensing for dynamic contrast-enhanced MRI using regional spatiotemporal sparsity and region tracking: block low-rank sparsity with motion-guidance (BLOSM). Magn Reson Med, 2014. 72(4): p. 1028-38.
4. Cernicanu, A. and L. Axel, Theory-based signal calibration with single-point T1 measurements for first-pass quantitative perfusion MRI studies. Acad Radiol, 2006. 13(6): p. 686-93.
5. Jerosch-Herold, M., N. Wilke, and A.E. Stillman, Magnetic resonance quantification of the myocardial perfusion reserve with a Fermi function model for constrained deconvolution. Med Phys, 1998. 25(1): p. 73-84.
6. Naresh, N.K., et al., Repeatability and variability of myocardial perfusion imaging techniques in mice: Comparison of arterial spin labeling and first-pass contrast-enhanced MRI. Magn Reson Med, 2015.
Figures