0547
Free-breathing Black-blood Prepared Cardiac Diffusion Tensor Imaging1Institute for Biomedical Engineering, ETH and University Zurich, Zurich, Switzerland
Synopsis
In vivo cardiac Diffusion Tensor Imaging (DTI) can be influenced by myocardial perfusion. To address this limitation, reference images with moderate diffusion weighting can be employed, which, however, reduce diffusion contrast and require the acquisition of three orthogonal diffusion encoding directions. In this work, blood suppression was implemented using black blood preparation to reduce contribution of perfusion to the diffusion weighted signal. This allows for the use of a marginally weighted reference image resulting in a reduction in scan time by about 17% with improved or comparable accuracy of diffusion metrics relative to previous methods.
Introduction
In vivo cardiac Diffusion Tensor Imaging (DTI) is challenging due to physiological factors including cardiac and respiratory motion and tissue perfusion. These limitations can in part be addressed by motion compensated diffusion gradient waveforms1-3, dedicated ECG triggering4, post-processing5,6, respiratory navigator gating7 and optimized b-value setting8. To mitigate perfusion effects9, reference images with a moderate diffusion weighting (e.g. b=100s/mm2) have been proposed10. This approach, however, requires three orthogonal diffusion encoding directions to ensure reference homogeneity and reduces diffusion contrast relative to the high diffusion weighted images (e.g. b=450s/mm2). The objective of the present work was to implement and test a black-blood preparation scheme for motion compensated spin echo (SE) DTI to reduce the effects of perfusion on diffusion metrics. In vivo data were acquired in the healthy human heart with a time efficient single reference image (b=20s/mm2) and diffusion metrics were compared to conventional acquisitions methods.Methods
Data acquisition
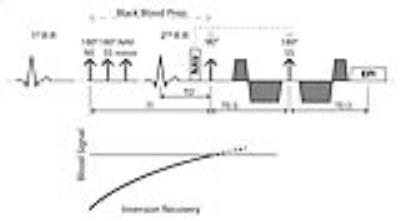
A cardiac-triggered diffusion-weighed second-order motion compensated SE sequence1 was employed on a 1.5T Philips Achieva system (Philips Healthcare, Best, The Netherlands) equipped with a 32-channel cardiac receiver array and a gradient system delivering 80mT/m @ 100mT/m/ms. Data were acquired in 4 healthy subjects (3 female, weight 66±14kg, age 25±2years, heart rate 59±7beats/min). Written informed consent according to institutional guidelines was obtained prior to imaging. Fat suppression was performed using a 1-3-3-1 binomial spatial-spectral excitation pulse11 selected along phase encoding direction for reduced field-of-view imaging. A variable rate selective excitation (VERSE)12 echo pulse was employed to reduce echo times (TE). Imaging was triggered to 50% end systole (TD). Blood suppression was achieved by a black-blood (BB) dual-inversion scheme13: A non-selective inversion pulse (NS) was played out in mid diastole followed by a slice-selective reinversion pulse (SS, Figure 1). The inversion time TI was calculated according to: $$TI=-T1\cdot log(\frac{1+exp(-\frac{TR}{T1})}{2})$$
with T1 of blood (1441ms14) and TR as derived from the subject’s heart rate (HR): $$$TR[ms] = 2 \cdot \frac{60 bpm}{HR[bpm]}\cdot 1000 [ms]. $$$ For the proposed BB approach, diffusion encoding was applied along 9 (b=450s/mm2) directions and 1 reference image with a b-value of 20s/mm2. Diffusion imaging with identical imaging parameters and three reference images (b=100s/mm2, encoded along orthogonal directions10), but without BB preparation was performed subsequently (Figure 2). Data acquisition was performed during free breathing with slice tracking based on a respiratory navigator placed on the right hemi-diaphragm15. Imaging parameters were: in-plane resolution 2.5×2.5mm2, slice thickness 8mm, FOV: 230×98mm2, TE/TR: 67ms/2-R-R. Number of signal averages: 8.
Data postprocessing and analysis
Prior to diffusion tensor calculation, geometric consistency along diffusion encoding directions was ensured by non-rigid image registration16. DTI analysis was performed on mean diffusivity (MD), fractional anisotropy (FA), the local helix elevation (helix angle), the deviation of the helix from circumferential contour (transverse angle)17 and the E2A sheet angle18. The root mean square error (RMSE) of linear regression of the transmural helix angle distribution was calculated. SNR analysis was performed in the left ventricle on both reference images, i.e. with and without BB preparation (b=20 and 100s/mm2, respectively).
Results
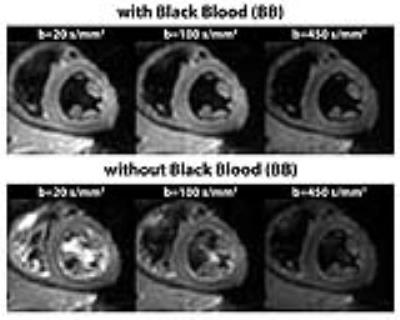
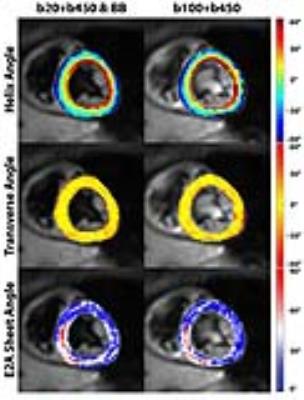
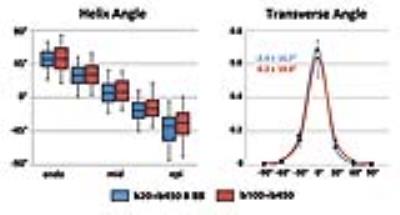
Scan time of the proposed BB approach was reduced by 17% compared to previous acquisition methods (total images: 10 vs. 12). Effective blood suppression can be appreciated in Figure 3. A comparison of helix, transverse and sheet angle maps acquired in the same subject is given in Figure 4. The linear transmural change of helix angles was found to be similar for both techniques as shown in Figure 5. Transverse angles were close to zero degrees with significantly decreased standard deviation in the BB case (-2.4±16.7° vs. 0.2±19.0°) (Figure 5). The RMSE of transmural helix angles was lower for BB (16.6±4.9° vs. 17.1±2.6°). FA values obtained with and without BB preparation were comparable (0.35±0.04 vs. 0.34±0.01) while MD values were lower with blood suppression (1.40±0.10x10-3mm2/s vs. 1.47±0.05x10-3mm2/s). SNR of the b=20s/mm2 image was increased by 15% relative to the b=100s/mm2 acquisition (24.0±2.2 vs. 20.8±1.6).Discussion
Black blood prepared cardiac DTI using second-order motion-compensated SE DTI allows for shorter scan times (~17%) compared to previous acquisition schemes10. Additionally, diffusion tensor accuracy is improved by increased diffusion contrast relative to the reference image and the increased reference SNR. Alternatively, keeping the diffusion contrast constant, the maximum b-value can be reduced by ~80-100s/mm2 which results in a reduction in TE of 3ms that translates into a theoretical SNR increase of ~6%.Conclusion
Black blood prepared cardiac DTI reduces the impact of tissue perfusion on diffusion metrics which can be exploited for a more favorable diffusion encoding scheme with respect to scan time and tensor accuracy.Acknowledgements
No acknowledgement found.References
1. Stoeck CT, von Deuster C, Genet M, Atkinson D, Kozerke S. Second Order Motion Compensated Spin-Echo Diffusion Tensor Imaging of the Human Heart. Magn Reson Med 2015. doi: 10.1002/mrm.25784.
2. Welsh C, Di Bella E, Hsu E. Higher-Order Motion-Compensation for In Vivo Cardiac Diffusion Tensor Imaging in Rats. IEEE Trans. Med. Imaging 2015;0062:1–1. doi: 10.1109/TMI.2015.2411571.
3. Gamper U, Boesiger P, Kozerke S. Diffusion imaging of the in vivo heart using spin echoes--considerations on bulk motion sensitivity. Magn Reson Med 2007;57:331–337.
4. Tseng W, Reese T. Cardiac diffusion tensor MRI in vivo without strain correction. Magn. Reson. Med. 1999;403:393–403.
5. Stoeck CT, Kalinowska A, von Deuster C, Harmer J, Chan RW, Niemann M, Manka R, Atkinson D, Sosnovik DE, Mekkaoui C, Kozerke S. Dual-phase cardiac diffusion tensor imaging with strain correction. PLoS One 2014;9:e107159.
6. Pai VM, Rapacchi S, Kellman P, Croisille P, Wen H. PCATMIP: enhancing signal intensity in diffusion-weighted magnetic resonance imaging. Magn Reson Med 2011;65:1611–1619.
7. Nielles-Vallespin S, Mekkaoui C, Gatehouse P, Reese TG, Keegan J, Ferreira PF, Collins S, Speier P, Feiweier T, Silva R. In vivo diffusion tensor MRI of the human heart: Reproducibility of breath-hold and navigator-based approaches. Magn. Reson. Med. 2013;70:454–465.
8. Scott AD, Ferreira PF, Nielles-Vallespin S, Gatehouse P, McGill L-A, Kilner P, Pennell DJ, Firmin DN. Optimal diffusion weighting for in vivo cardiac diffusion tensor imaging. Magn. Reson. Med. 2015;74:420–430.
9. Le Bihan D. Intravoxel incoherent motion imaging using steady-state free precession. Magn. Reson. Med. 1988;7:346–51.
10. Von Deuster C, Stoeck CT, Genet M, Atkinson D, Kozerke S. Spin echo versus stimulated echo diffusion tensor imaging of the in vivo human heart. Magn. Reson. Med. 2015;00:n/a–n/a. doi: 10.1002/mrm.25998.
11. Meyer CH, Pauly JM, Macovski A, Nishimura DG. Simultaneous spatial and spectral selective excitation. Magn. Reson. Med. 1990;15:287–304.
12. Hargreaves BA, Cunningham CH, Nishimura DG, Conolly SM. Variable-rate selective excitation for rapid MRI sequences. Magn. Reson. Med. 2004;52:590–597.
13. Edelman R, Chien D, Kim D. Fast selective black blood MR imaging. Radiology 1991;181:655–660.
14. Montant P, Sigovan M, Revel D, Douek P. MR imaging assessment of myocardial edema with T2 mapping. Diagn Interv Imaging 2015.
15. Moulin K, Croisille P, Feiweier T, Delattre BM a, Wei H, Robert B, Beuf O, Viallon M. In-vivo free-breathing DTI & IVIM of the whole human heart using a real-time slice-followed SE-EPI navigator-based sequence: a reproducibility study in healthy volunteers. Magn Reson Med 2015. doi: 10.1002/mrm.25852.
16. Vishnevskiy V, Gass T, Szekely G, Tanner C, Goksel O. Isotropic Total Variation Regularization of Displacements in Parametric Image Registration. IEEE Trans. Med. Imaging 2016;0062:1–1. doi: 10.1109/TMI.2016.2610583.
17. Scollan DF, Holmes A, Winslow R, Forder J. Histological validation of myocardial microstructure obtained from diffusion tensor magnetic resonance imaging. Am J Physiol 1998;275:H2308–18.
18. Ferreira P, Kilner PJ, McGill L-A, Nielles-Vallespin S, Scott AD, Spottiswoode BS, Zhong X, Ho SY, McCarthy K, Ismail T, Gatehouse P, Silva R, Lyon A, Prasad SK, Firmin D, Pennell DJ. In vivo cardiovascular magnetic resonance diffusion tensor imaging shows evidence of abnormal myocardial laminar orientations and mobility in hypertrophic cardiomyopathy. J. Cardiovasc. Magn. Reson. 2014;16:P338.
Figures