0541
Inducibility of ventricular arrhythmia correlates with the indices of myocardial viability using manganese enhanced MRI (MEMRI) in a porcine ischemia reperfusion model1Cardiovascular Medicine, Stanford University, Stanford, CA, United States, 2Radiology, AIC Yaesu Clinic, Tokyo, Japan, 3Graduate School of Human Health Sciences, Tokyo Metropolitan University, Tokyo, Japan
Synopsis
Peri-infarct region (PIR), containing the viable but injured myocardium, has been related to the occurrence of ventricular arrhythmia. Reliable in vivo detection of arrhythmogenic region presents significant challenge. While delayed enhanced MRI (DEMRI) with gadolinium (Gd) detects the myocardial infarction, the non-specific property does not detect the viable but injured cardiomyocytes in the PIR. Manganese (Mn) enters the live cells via L-type calcium channel, and enables dual enhancement technique to identify the overlapping viable region in PIR. We measured the correlation between the volume of the PIR and inducibility of ventricular arrhythmia using porcine ischemia reperfusion model.
Introduction
The presence of viable cells within the myocardial scar, identified as the peri-infarct region (PIR), could produce a substrate for ventricular tachyarrhythmia (VT).1 A reliable non-invasive detection of this arrhythmogenic region presents significant challenges. Gadolinium (Gd)-based delayed enhanced magnetic resonance imaging (DEMRI) is the current gold standard in evaluating the irreversible myocardial injury. However, DEMRI does not provide direct cell viability data because of its nonspecific distribution within the extracellular space. In contrast, manganese (Mn) enters live myocardium via L-type calcium channel, providing a mechanism to directly visualize and quantify the myocardial viability through a strong T1 shortening effect. Dual enhancement technique using DEMRI and Mn-enhanced MRI (MEMRI) identified the overlapping areas in the PIR consisting of injured but viable myocardium.2 Precise characterization of this region may have far-reaching implications. The extent of myocardial injury in the PIR is predicted to correlate with ventricular arrhythmia and remodeling. The tissue heterogeneity in the PIR and the subsequent fibrosis in the remote region may determine the arrhythmia risk.Purpose
The purpose of this study is to test the hypothesis that the volume of viable myocardium in the PIR correlates with the likelihood of developing ventricular arrhythmia in a porcine ischemia reperfusion (IR) injury model.Methods
Twenty female swine underwent left anterior descending (LAD) IR injury by a 60-minute balloon occlusion at the mid-LAD (N=8) or proximal-LAD (N=12). Four weeks post-IR, cardiac MRI (3T signa-HD, GE) was performed to assess the ejection fraction (EF), scar volume by DEMRI (0.2 mmol/kg of Gd-DTPA) and myocardial viability volume by MEMRI (0.7 ml/kg of EVP1001-1, Eagle Vision Pharmaceuticals) on left ventricular (LV) short axis. T1 mapping was performed at pre-contrast, post-Mn and post-Gd administration with Saturation Method using Adaptive Recovery Times for cardiac T1 Mapping (SMART1Map) sequence.4 T1 values and extra-cellular volume (ECV) were calculated in remote region. The programmed electrical stimulation (PES) from the right ventricular (RV) apex using burst pacing and extra-stimuli was performed, and we related the PIR indices to arrhythmia inducibility (baseline S1 - coupling interval at VT).Results
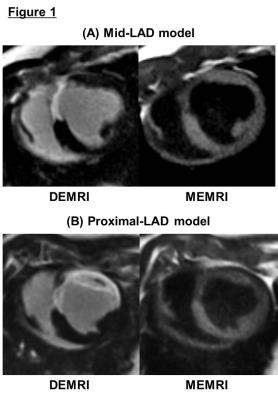
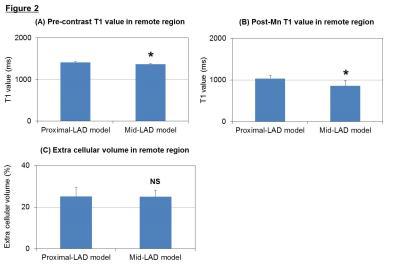
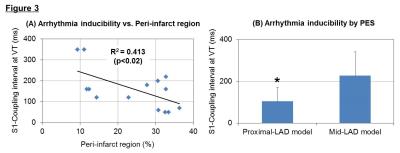
Myocardial infarction was reliably established in 20 pigs (weight 41.1±7.7 kg, LVEF due to mid-LAD stenosis: 34.1±3.7 %* and at proximal-LAD: 23.2±6.2%, *p<0.001). Measurement of % infarct volume by DEMRI exceeded MEMRI measurement, representing a significant over-estimation of the infarct size by DEMRI vs. MEMRI, respectively (mid-LAD: 15.2±5.0 %* vs. 7.7±3.1 %, proximal-LAD: 31.1±5.0 %* vs. 9.1±5.0 %, *p<0.003), demonstrating significant viability in the PIR (mid-LAD: 7.5±2.8 %*, proximal-LAD: 20.8±7.6 %, *p<0.001) (Fig 1). The difference in infarct size had an effect on the T1 value in remote region, mid-LAD model demonstrated significantly greater T1 shortening value when compared to the proximal-LAD model both in pre-contrast (mid-LAD: 1363.7±15.6 ms* vs. proximal-LAD: 1411.8±20.7 ms, *p<0.003) and post-Mn injection (mid-LAD: 858.7±128.3 ms* vs. proximal-LAD: 1034.9±74.1 ms, *p<0.008) (Fig 2-A,B). However, the assessment of ECV did not show any significant difference between the two groups (mid-LAD: 25.0±3.1 % vs. proximal-LAD: 25.0±4.4 %, NS) (Fig 2-C). These data indicated the progress of microscopic fibrosis and downregulation of myocardial function in remote region for the proximal-LAD model. The lower threshold for burst pacing and extra-stimuli to induce VT correlated strongly with the % volume of PIR (R2=0.413, p<0.02) (Fig 3-A). The proximal-LAD model demonstrated significantly shorting of baseline S1 - coupling interval at VT when compared to the mid-LAD model (mid-LAD: 228±112.6 ms vs. proximal-LAD: 104.3±65.5 ms*, *p<0.04), suggesting more arrhythmia inducibility in proximal-LAD model (Fig 3-B).Discussion
The proximal-LAD model demonstrated significantly increased PIR and risk for the arrhythmia inducibility when compared to the mid-LAD model. The significantly prolonged T1 value at pre-contrast is considered as the increase of fibrosis and edema of LV in proximal-LAD model.3 Although ECV differences were undetectable between the two groups, suggesting that the process of fibrosis is still early stage and minute differences cannot be distinguished by Gd-contrast enhancement. In contrast, the uptake of Mn is correlated with the function of myocardial viability, thus the longer T1 value observed in proximal-LAD model post-Mn injection suggests the downregulation of myocardial status. Such structural alterations in the remote myocardium may indicate the mechanism of the increased arrhythmia inducibility.Conclusion
Using novel MEMRI indices, we demonstrated a significant correlation between MEMRI measurement of PIR viability and ventricular arrhythmia inducibility by PES in a porcine IR model. These data are the first to associate the viable morphology and LV scar anatomy at high spatial resolution in the PIR with potential implications for the detection of clinical arrhythmia risk.Acknowledgements
No acknowledgement found.References
1. Schmidt A, Azevedo CF, Cheng A, et al. Infarct tissue heterogeneity by magnetic resonance imaging identifies enhanced cardiac arrhythmia susceptibility in patients with left ventricular dysfunction. Circulation 2007;115(15):2006-2014.
2. Dash R, Chung J, Ikeno F, et al. Dual manganese-enhanced and delayed gadolinium-enhanced MRI detects myocardial border zone injury in a pig ischemia-reperfusion model. Circ Cardiovasc Imaging 2011;4(5):574-582.
3. Chan W, Duffy SJ, White DA, et al. Acute left ventricular remodeling following myocardial infarction: coupling of regional healing with remote extracellular matrix expansion. JACC Cardiovasc Imaging 2012;5(9):884-893.
4. Slavin G, Stainsby J. True T1 Mapping with SMART1Map: A Comparison with MOLLI. ISMRM 2013, p.1416.
Figures