0535
pH Measurement of Intervertebral Disc in the Process of Disc Degeneration Using Quantitative Chemical Exchange Saturation Transfer (qCEST)1Biomedical Imaging Research Institute, Cedars-Sinai Medical Center, Los Angeles, CA, United States, 2Department of Bioengineering, University of California, Los Angeles, 3Skeletal Biotech Laboratory, Hebrew University of Jerusalem, Israel, 4BOG Regenerative Medicine Institute, Cedars-Sinai Medical Center, Los Angeles, CA, United States, 5Department of Surgery, Cedars-Sinai Medical Center, Los Angeles, CA, United States, 6Department of Imaging, Cedars-Sinai Medical Center, CA, United States, 7Department of Orthopedics, Cedars-Sinai Medical Center, Los Angeles, CA, United States
Synopsis
Several hypotheses associate the pathogenesis of discogenic low back pain with low pH. In this study, we used qCEST MRI technique to monitor the pH changes of the intervertebral disc in the process of degeneration in porcine model. A significant pH drop was observed at 2 weeks following degeneration induction. This trend is correlated with the expression of pain markers. These results suggest that qCEST MRI has the potential to serve as a novel and non-invasive method for the diagnosis of discogenic pain.
Background
Intervertebral disc (IVD) degeneration is one of the leading causes of chronic low back pain. The current clinical MRI provides detailed tissue anatomy, but fails to differentiate between a pathologically painful disc and a physiologically aging disc that does not generate pain. Several hypotheses associate the pathogenesis of discogenic low back pain with low pH1.
Chemical exchange saturation transfer (CEST) is a technique that can be used to detect GAG content in the IVD and pH value is also one important factor that affects the CEST signal2,3. However, CEST is a relatively complex system, with multiple confounding factors like exchange rate (pH related), labile proton fraction (concentration related), T1 and T2. Quantitative CEST (qCEST) is an emerging technique that allows full determination of labile proton ratio (concentration related) and exchange rate (pH related)4,5. Previous work has shown the feasibility of pH measurement of IVD using qCEST6.
In this study, we applied the qCEST technique to monitor the pH changes in the process of disc degeneration in porcine model. The pH values were also compared with the expression of pain markers.
Methods
Theory: Recent studies have shown that in a CEST experiment with Gaussian saturation pulses, the inverse CEST difference can be described as4,5
$$\frac{1}{CESTR_{ind}}=\frac{1}{\frac{1}{Z_{label}}-\frac{1}{Z_{ref}}}\approx\frac{R_{1w}}{DC\cdot f_{r}\cdot k_{sw}\cdot c_{1}}+\frac{k_{sw}\cdot(R_{2s}+k_{sw})\cdot R_{1w}\cdot c_{2}^2}{DC\cdot f_{r}\cdot k_{sw}\cdot c_{1}}\cdot\frac{1}{\omega_{1}^2}$$
with exchange rate ksw, labile proton fraction fr = M0s/M0w, RF irradiation amplitude ω1, water R1w and solute protons R2s. DC represents the duty cycle and c1 and c2 represent the shape of Gaussian saturation pulses ($$$c_{1}=\sigma\sqrt{2\pi}/t_{p}, c_{2}=\sqrt{\sqrt{2}};$$$ σ and tp are the width and length of the Gaussian pulse). Zlabel and Zref are the normalized CEST signal at +1.0 ppm and -1.0 ppm, respectively for GAG. In this expression, 1/CESTRind and 1/ω12 are described as linear regression. By measuring CESTRind with different RF irradiation amplitude ω1, we can calculate the slope and intercut, and eventually estimate ksw and fr.
Animal Handling: All animal procedures were approved by the local institutional review board for animal experiments (IACUC). Nine Yucatan mini-pigs were used in this study. Disc punctures were performed in 3 levels in order to simulate IVD degeneration. MRI studies were performed at 2, 6 and 10 weeks after disc degeneration. Pain, nerve and inflammatory gene expression was evaluated using qRT-PCR and validated using immunohistochemistry (IHC) done on harvested IVDs at 2, 6 and 10 weeks after degeneration.
Imaging Protocol: Imaging experiments were conducted on a 3T Siemens Verio clinical scanner. All CEST scans were performed in the axial plane using single-shot reduced-FOV TSE readout (FOV: 140 x 40 mm2; resolution: 1.1 x 1.1 x 3 mm3; matrix size: 128 x 38, number of averages: 2). CEST preparation module consists of 39 Gaussian pulses with duration of 80 ms and duty cycle of 50%. Total saturation time is 6240 ms to ensure CEST event has reached steady state. CEST images were acquired at 10 different saturation frequency offsets (±1.6, ±1.3, ±1.0, ±0.7, ±0.4 ppm). Four different RF irradiation amplitudes B1rms were chosen: 0.63, 1.05, 1.47 and 1.89 uT (corresponding flip angles of single Gaussian pulse were 900°, 1500°, 2100° and 3000°). B0 map was acquired using WASSR.
Data Analysis: Region-of-interest (ROI) was placed to include nucleus pulposus of each disc. Exchange rate map was generated pixel by pixel within the ROI as described in theory. Average exchange rate of each IVD was also generated within the ROI. The exchange rate was converted to pH based on the relationship established in the previous study6.
Statistical analysis was conducted using a one-way ANOVA with Tukey’s multiple comparison post hoc test. p < 0.05 was considered statistically significant.
Results
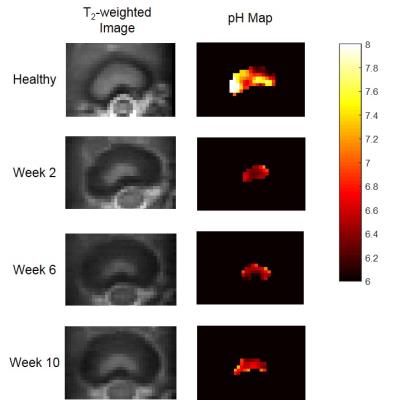
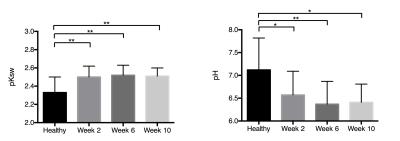
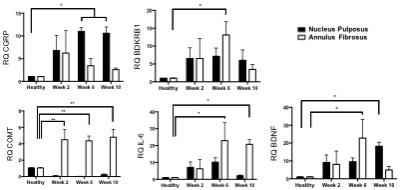
Fig.1 represents the T2-weighted axial anatomy images and pH maps acquired at different time points of the degeneration process. A significant drop in the intra-discal pH values was observed at 2 weeks following degeneration induction (p < 0.05, Fig. 2). Expression analysis of genes from degenerated IVDs revealed significant upregulation of pain-, nerve- and inflammatory-related genes at 6 and 10 weeks after degeneration (Fig. 3), which was validated by immunofluorescence. These markers were also found to be upregulated in patients suffering from discogenic pain, supporting the hypothesis that acidic environment in degenerative IVDs could induce pain.Conclusion
This study shows that qCEST MRI can detect pH changes within the IVD in the process of disc degeneration in porcine model. This trend is correlated with the expression of pain markers. These results suggest that qCEST MRI has the potential to serve as a novel and non-invasive method for the diagnosis of discogenic pain.Acknowledgements
No acknowledgement found.References
1. Liang, et al. "The relationship between low pH in intervertebral discs and low back pain: a systematic review." Arch Med Sci 8.6 (2012): 952-6.
2. Liu, et al. "Detection of low back pain using pH level-dependent imaging of the intervertebral disc using the ratio of R1ρ dispersion and− OH chemical exchange saturation transfer (RROC)." Magn Reson Med 73.3 (2015): 1196-1205.
3. Melkus, et al. "Ex vivo porcine model to measure pH dependence of chemical exchange saturation transfer effect of glycosaminoglycan in the intervertebral disc." Magn Reson Med 71.5 (2014): 1743-1749.
4. Wu, et al. "Quantitative chemical exchange saturation transfer (qCEST) MRI–omega plot analysis of RF-spillover-corrected inverse CEST ratio asymmetry for simultaneous determination of labile proton ratio and exchange rate." NMR in Biomed 28.3 (2015): 376-383.
5. Meissner, et al. "Quantitative pulsed CEST-MRI using Ω-plots." NMR in Biomed 28.10 (2015): 1196-1208.
6. Zhou, et al. "Quantitative chemical exchange saturation transfer MRI of intervertebral disc in a porcine model." Magn Reson Med (2016).
Figures