0532
Combined Late Gadolinium Enhancement (LGE) and 18F-fluorodeoxyglucose (18F-FDG) Uptake in a Hybrid PET/MR System to Diagnose Active Cardiac SarcoidosisPhilip M Robson1, Maria Giovanna Trivieri2, Ronan Abgral3, Marc R Dweck4, Nicolas A Karakatsanis1, Venkatesh Mani1, Maria M Padilla5, Marc M Miller6, Anarahda Lala6, Javier Sanz6, Jagat Narula6, Valentin Fuster6, Johanna Contreras6, Jason Kovacic6, and Zahi A Fayad1
1Translational and Molecular Imaging Institute, icahn school of medicine at mount sinai, New York, NY, United States, 2icahn school of medicine at mount sinai, New York, NY, United States, 3Department of Nuclear Medicine, European University of Brittany, 4British Heart Foundation/University Centre for Cardiovascular Science, University of Edinburgh, 5Division of Pulmonary, Critical Care and Sleep Medicine, icahn school of medicine at mount sinai, New York, NY, United States, 6Cardiovascular Institute, icahn school of medicine at mount sinai, New York, NY, United States
Synopsis
Recent advances in hybrid Positron Emission Tomography (PET) Magnetic Resonance (MR) technology have enabled simultaneous imaging with both modalities. Sarcoidosis is a granulomatous disease that, when involving the heart has a poor prognosis. However, cardiac sarcoidosis has been shown to respond to immunosuppressive therapy. Currently, both late gadolinium enhancement (LGE)-MR and 18F-fluorodeoxyglucose (18F-FDG)-PET are used separately to evaluate the disease yet a clear diagnosis is not easily achieved. In this work, we investigate the potential improvement in evaluation with combined 18F-FDG-PET/MR imaging.
Introduction
Sarcoidosis is a granulomatous disease of unknown etiology that most commonly affects the lungs and mediastinal lymph nodes. Heart involvement poses an increased risk of sudden death but is probably under-diagnosed due to frequent absence of clinical symptoms and the inability to easily diagnose cardiac sarcoidosis (CS) with a non-invasive method [1-4]. Increased inflammation associated with CS can be characterized on 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET) [5] whereby metabolically active macrophages associated with inflammation take up 18F-FDG, a glucose analogue, more avidly than surrounding tissue. However, the myocardium also uses glucose as an energy source and frequently, pathological uptake can be masked by physiological uptake. CS can also be characterized by the pattern of myocardial injury seen on late gadolinium enhancement (LGE) magnetic resonance (MR) imaging [6]. The recent advances in hybrid PET/MR systems now allow simultaneous evaluation of patients with PET and MR. Our aim was to assess the usefulness of combined LGE-MR imaging and 18F-FDG-PET in the diagnosis of CS by using areas of LGE to guide the evaluation of 18F-FDG uptake.Methods
Patients with previous history of biopsy proven extra-cardiac sarcoidosis and/or clinical symptoms consistent with CS were referred to our department (Translational and Molecular Imaging Institute, Mount Sinai Hospital, New York) for PET/MR imaging (Biograph mMR, Siemens). LGE-MR images were acquired with breath-held multi-slice short-axis imaging using an inversion-recovery segmented fast low-angle shot (FLASH) acquisition with ECG triggering and diastolic acquisition, 10-15 minutes after injection of 0.2 mmol/kg gadolinium contrast agent (Multihance, Bracco, Milan). The inversion time was determined by TI-scout. In addition, T2-mapping was performed with breath-held multi-slice short-axis images using an ECG-triggered FLASH acquisition with T2-prep (Siemens WIP 448). PET data were acquired in list-mode format over 90 minutes post injection of approximately 370 MBq of 18F-FDG, with the final 30 minutes used in the following analysis. PET images were reconstructed using an attenuation map estimated by segmentation of a 3D breath-held DIXON-VIBE MR sequence into background, lung, fat and soft tissue, and using an iterative OP-OSEM algorithm with 3 iterations and 21 subsets. For image analysis, the following quantitative parameters were measured: mean and maximal myocardial standardized uptake values (SUVmax, SUVmean) around areas positive for LGE, if present; mean T2 mapping value (T2mean) in the same volume; target-to-blood pool ratios (TBRmax, TBRmean); and target-to-negative LGE myocardium ratios (TNRmax, TNRmean). A final diagnosis of active cardiac sarcoidosis (CS+) or not active (CS-) was defined by a consensus of clinical experts with access to all clinical, imaging and biopsy data. Mean values of different imaging parameters in CS+ and CS- patients were compared using a Student t-test and a ROC analyses was performed to assess diagnostic accuracy of imaging parameters.Results
Twenty-six patients (12M/14F; 55.2±9.7 yo) were prospectively selected from August 2015 to April 2016. One of them did not perform the exam due to claustrophobia. All others underwent PET/MR. Eight patients were considered as CS+ and 17 as CS-. Co-localization of 18F-FDG uptake and LGE in a patient considered CS+ and the absence of LGE in a patient with avid 18F-FDG uptake considered CS- are shown in Fig. 1. There was no statistically significant difference of mean SUVmax (p=0.266), T2mean (p=0.320), TBRmax (p=0.239) and TBRmean (p=0.248) in CS+ and CS- patients. Mean TNRmax were respectively 1.68±0.41 and 1.09±0.08 in CS+ and in CS- patients (p<0.0001). Mean TNRmean were respectively 1.52±0.27 and 1.03±0.14 in CS+ and in CS- patients (p<0.0001). ROC analysis revealed a threshold of TNRmax=1.21 (AUC=0.978) to differentiate all patients as being CS+ or CS- with a diagnostic accuracy of 96% (Fig. 2). Two previously unknown cases of sarcoid involvement in bone and in liver were also identified. In the CS- group, combined PET/MR identified an alternative cause for cardiac symptoms in 6 patients (1 arrhythmogenic right ventricular cardiomyopathy, 1 incidental chronic infarction, 1 aortic valve fibroelastoma, 1 old scar of inflammatory disease, 2 with anomalous coronary origin with malignant course).Conclusion
The results of our prospective study show the usefulness of combined PET/MR in the diagnosis of CS using TNR measurements, which are based on identifying the area of inflammation on 18F-FDG-PET images by selecting regions of LGE-MR. Such a measurement is facilitated by the simultaneous measurement of MR and PET with a hybrid system. This series also confirms the clinical ability of PET/MR imaging to evaluate extra-cardiac involvement of sarcoidosis and in assessing alternative myocardial pathologies.Acknowledgements
This work was supported by NIH grant R01 HL071021References
[1] Iannuzzi et al. N Engl J Med 2007. [2] Silveman et al. Circulation 1978. [3] Newma et al. N Engl J Med 1997. [4] Birnie et al. JACC 2016. [5] Youssef et al. J Nucl Med 2012. [6] Greulich et al. JACC cardiovascular imaging 2013.Figures
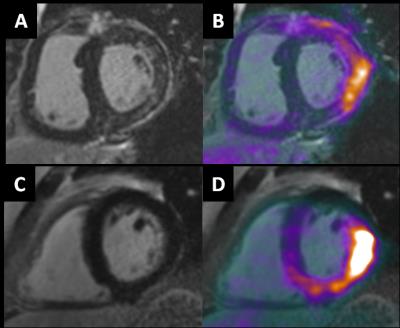
Figure 1: In
patient 1, considered to show positive cardiac sarcoidosis (CS+),
characteristic LGE (A) co-localizes with 18F-FDG uptake indicating active
disease. In patient 2, considered
negative for cardiac sarcoidosis (CS-), no LGE is present (C) suggesting
physiological 18F-FDG uptake (D).
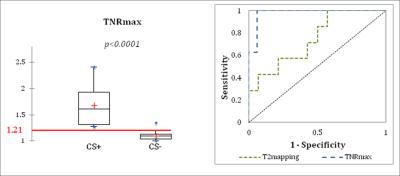
Figure 2: Results
of TNRmax measurements, where regions of LGE-MR are used to guide 18F-FDG
measurements. A cut-off value in TNRmax
of 1.21 separates patients determined to be CS+ from CS- (left); ROC analysis
shows high sensitivity (100%), specificity (94%), AUC (0.978) and diagnostic
accuracy (96%) for TNRmax measurements in cardiac sarcoidosis.