0531
Intravoxel Incoherent Motion Model in the Heart of Patients under Adenosine Induced Stress1Institute for Biomedical Engineering, ETH Zürich, Zürich, Switzerland, 2Cardiology, University Hospital Zürich
Synopsis
Intravoxel Incoherent Motion Imaging (IVIM) in the in vivo human heart has the potential of measuring myocardial perfusion without the need for contrast agents. In order to validate previous IVIM animal studies, patients where measured both during rest and under adenosine induced stress using a slice following second-order motion compensated diffusion weighted imaging sequence. The IVIM perfusion fraction is found to significantly increase during stress, which shows that IVIM imaging allows measuring a perfusion surrogate. This can hence be used for example to assess perfusion deficits in patients with ischemia.
Introduction
In recent years, the application of the Intravoxel Incoherent Motion (IVIM) model (1) in the heart (2, 3) has gained rising interest, because of its ability to provide a contrast agent free perfusion estimate. The IVIM model has also been applied to other organs in numerous studies (4, 5). A study in canine hearts (6) has found an increased IVIM perfusion estimate under adenosine administration.
The objective of the present work was to validate the increased IVIM perfusion surrogate in patients under adenosine influence by using a slice following second-order motion compensated diffusion weighted sequence.
Methods
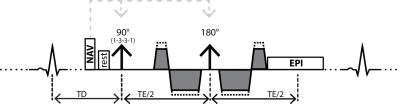
A second-order motion compensated diffusion weighted spin-echo EPI sequence (Figure 1) (7) was used on a 1.5T Philips Achieva system (Philips Healthcare, Best, The Netherlands) equipped with a 5-channel cardiac receiver coil array and a gradient system delivering 80mT/m at 100mT/m/ms.
Data from five patients (2 arterial hypertonia, 1 diabetes mellitus, 1 overweight, 1 heart transplant, 5 male, mean age 55±8years, mean weight 77±10kg, mean heart rate 64±11bpm (rest), respectively 79±12bpm (stress)) were acquired. Three short-axis slices at basal, mid-ventricular and apical level were prescribed and diffusion images were obtained with following parameters: spatial resolution: 2.4×2.4mm2, slice thickness: 10mm, slice gap: 10mm, reduced field-of-view (FOV): 230×105mm2, TR/TE: 2R-R/99ms, spectral-spatial water-only excitation. Diffusion encoding was performed using 15 b-values (range: 0-300s/mm2) acquired along six diffusion encoding directions (8) during cardiac contraction (50% end systole). Imaging was performed during free breathing with automatic slice tracking by using a navigator pencil beam on the right hemi-diaphragm. First, a baseline scan during rest was performed. Second, adenosine was administered (59±8mg). After 30s, the second acquisition was performed during constant adenosine flow (140μg/kg/min) until the end of the measurement. The total scan time of one acquisition was 4:15min at a heart rate of 60bpm.
In post-processing, image registration of diffusion weighted images was performed (9) to correct for residual respiratory motion induced geometrical inconsistency. In addition, image intensities were corrected to account for variations of the effective repetition time TR as a result of varying heart rate using literature values for T1 of the myocardium (10). Complex averaging of the respective diffusion directions was performed (11). For parameter regression, a modified segmented least-squares approach was implemented (12) in Matlab (Natick, MA). The myocardium was segmented into 4 (apical), 6 (mid-ventricular) and 6 (basal) sectors according to the AHA scheme (13). The mean signal S(b) of the voxels in one sector was fitted using the IVIM model in Eq. [1] with reference intensity S0, diffusion encoding strength b, diffusion coefficient D, perfusion fraction f and pseudo-diffusion coefficient D*:
$$S(b)=S_0 \left[f\cdot exp(-bD^{*})+(1-f)\cdot exp(-bD)\right] \qquad [1]$$
Mean values of the IVIM parameters and ranges are reported for the study population together with P-values from Mann-Whitney-U-Tests for the IVIM parameters comparing rest to stress acquisition.
Results
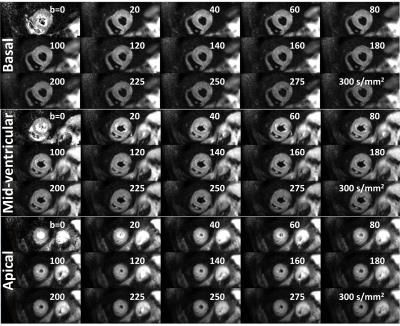
Systolic IVIM data
were successfully acquired in all subjects. Example magnitude images are shown
in Figure 2.
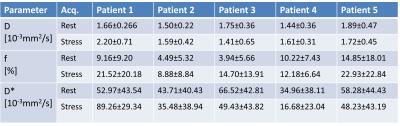
IVIM parameters of the individual patients are
displayed in Figure 3. Every patient exhibits an increase in perfusion fraction
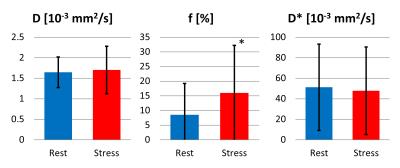
f. The diffusion coefficient D across all volunteers and sectors (Figure 4) was
1.65±0.38·10-3mm2/s (rest),
1.71±0.58·10-3mm2/s (stress), perfusion fraction f was 8.53±10.77%
(rest), 16.04±16.30% (stress) and pseudo-diffusion coefficient D* was 51.29±42·10-3mm2/s (rest),
47.82±42.97·10-3mm2/s (stress). Hence,
the diffusion coefficient remains within 5% relative difference among the two
acquisitions, while the perfusion fraction increases by about 88% under
adenosine. The pseudo-diffusion coefficient remains within 7% relative
difference between the two measurements. The perfusion fraction is
significantly different under stress (P=0.014),
while the diffusion and pseudo-diffusion coefficient are not (P=0.65, respectively P=0.25).Discussion
Successful mapping of IVIM parameters in the diseased human heart during systolic contraction was enabled by a second-order motion compensated diffusion weighted imaging sequence. The found IVIM parameters in Figure 4 are in line with literature values (2). A systolic data acquisition yields a thicker myocardium and a higher reproducibility in the presence of heart rate variations compared to diastolic acquisitions. This is particularly important for the stress measurement, where the heart rate increases. Adenosine induced stress in patients leads to an increase in perfusion fraction f (ca. 88%), higher than the increase found in canines (ca. 25%) in (6).
Conclusion
This study yields further evidence that the IVIM model can detect perfusion changes in the human heart during a clinical protocol using adenosine. Hence, IVIM parameter mapping is considered a promising approach to map myocardial perfusion surrogates without the need for contrast agent administration. Potentially, it can be used to detect local perfusion deficits, e.g. in the diseased/infarcted human heart.
Acknowledgements
This work is supported by VPH-DARE@IT.References
1. Le Bihan D. Intravoxel incoherent motion imaging using steady-state free precession. Magn. Reson. Med. 1988;7:346–351.
2. Moulin KK, Croisille P, Feiweier T, Delattre BMA a, Wei H, Robert B, Beuf O, Viallon M. In vivo free-breathing DTI and IVIM of the whole human heart using a real-time slice-followed SE-EPI navigator-based sequence: A reproducibility study in healthy volunteers. Magn. Reson. Med. 2016;76:70–82.
3. Delattre BMA, Viallon M, Wei H, Zhu YM, Feiweier T, Pai VM, Wen H, Croisille P. In vivo cardiac diffusion-weighted magnetic resonance imaging: quantification of normal perfusion and diffusion coefficients with intravoxel incoherent motion imaging. Invest. Radiol. 2012;47:662–670.
4. Marzi S, Piludu F, Forina C, Sanguineti G, Covello R, Spriano G, Vidiri A. Correlation Study between Intravoxel Incoherent Motion MRI and Dynamic contrast-enhanced MRI in Head and Neck Squamous Cell Carcinoma: Evaluation in Primary Tumors and Metastatic Nodes. Magn. Reson. Imaging 2016.
5. Federau C, Maeder P, O’Brien K, Browaeys P, Meuli R, Hagmann P. Quantitative measurement of brain perfusion with intravoxel incoherent motion MR imaging. Radiology 2012;265:874–881.
6. Callot V, Bennett E, Decking UKM, Balaban RS, Wen H. In vivo study of microcirculation in canine myocardium using the IVIM method. Magn. Reson. Med. 2003;50:531–540.
7. Stoeck CT, von Deuster C, Genet M, Atkinson D, Kozerke S. Second-order motion-compensated spin echo diffusion tensor imaging of the human heart. Magn. Reson. Med. 2016;75:1669–1676.
8. Jones DK, Horsfield M a., Simmons A. Optimal strategies for measuring diffusion in anisotropic systems by magnetic resonance imaging. Magn. Reson. Med. 1999;42:515–525.
9. Vishnevskiy V, Gass T, Szekely G, Tanner C, Goksel O. Isotropic Total Variation Regularization of Displacements in Parametric Image Registration. IEEE Trans. Med. Imaging 2016;62:1–1.
10. Messroghli DR, Plein S, Higgins DM, Walters K, Jones TR, Ridgway JP, Sivananthan MU. Human myocardium: single-breath-hold MR T1 mapping with high spatial resolution--reproducibility study. Radiology 2006;238:1004–1012.
11. Scott AD, Nielles-Vallespin S, Ferreira PF, McGill L-A, Pennell DJ, Firmin DN. The effects of noise in cardiac diffusion tensor imaging and the benefits of averaging complex data. NMR Biomed. 2016;29:588–599.
12. Notohamiprodjo M, Chandarana H, Mikheev A, Rusinek H, Grinstead J, Feiweier T, Raya JG, Lee VS, Sigmund EE. Combined intravoxel incoherent motion and diffusion tensor imaging of renal diffusion and flow anisotropy. Magn. Reson. Med. 2015;73:1526–1532.
13. Gersh BJ, Maron BJ, Bonow RO, et al. 2011 ACCF/AHA Guideline for the Diagnosis and Treatment of Hypertrophic Cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the American As. J Am Coll Cardiol 2011;58:212-260.
Figures