0527
Free-breathing 3D whole-heart stress myocardial perfusion using compressed sensing1CBRU, Royal Brompton Hospital, London, United Kingdom, 2NHLI, Imperial College London, London, United Kingdom, 3UCAIR, University of Utah, UT, United States
Synopsis
A consistent, reliable, 3D myocardial first-pass perfusion sequence is developed using a stack-of-stars design and tested at both stress and rest during free-breathing. A compressed sensing algorithm is used to compensate for the high undersampling rates, including a modified form with temporal pixel reordering designed to better cope with respiratory motion. Reconstructions were successful, despite large respiratory motion, in all cases. An example of a perfusion defect in a coronary artery disease patient is presented, with confirmation from recent SPECT myocardial perfusion scintigraphy.
Background
Myocardial first-pass perfusion (FPP) can be extended by 3D imaging to whole-heart coverage in every cardiac cycle with consistent cardiac phase for all reconstructed images1. Technical advances have enabled such acquisitions, with recent comparisons in patient cohorts demonstrating strong potential2,3.
However, imaging is constrained by the extreme sequence acceleration required to cut 3D shot duration in each cycle for minimised intra-shot cardiac motion. In all previous clinical stress validations of 3D FPP techniques, breath-holding was required for accurate reconstruction of the highly reduced acquired k-space lines1. The breath-hold requirement at stress could reduce reliability as co-operation under stress is often more difficult.
Compressed sensing (CS) allows high accelerations of FPP but is also often based on assumed breath-holding – previous 3D FPP work with CS has been performed at rest. Furthermore, the ability of a CS reconstruction to accurately resolve an induced perfusion defect has yet to be demonstrated for 3D FPP, with potential concerns over the strong temporal weighting often used.
This work aims to demonstrate optimised 3D FPP imaging with a modified CS reconstruction algorithm in free-breathing subjects at both rest and stress, including a first demonstration in a coronary artery disease (CAD) patient.
Methods
16 3D FPP scans (4 stress, 12 rest) were acquired in 12 subjects under consent and with ethical approval. Each scan was performed during free-breathing, with no instructions issued to the subject, capturing first-pass of 0.1mmol/kg Gadobutrol with 140ug/kg/min adenosine for stress. A simple and consistent protocol was used with only modification of slab position and trigger delay required.
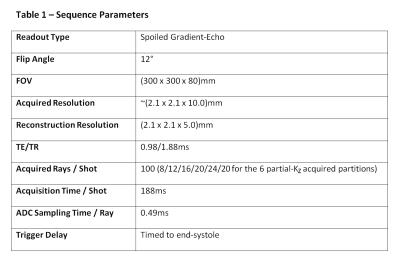
A 3D stack-of-stars sequence (Table 1) was utilised, combining the motion-tolerance and CS compatibility of in-plane radial acquisitions with the lower required sampling rate of Cartesian through-plane acquisition. The sequence was optimised with asymmetric echo (75%), slice partial Fourier (75%), and randomised RF spoiling4, to shorten TE/TR to 0.98/1.88ms, further combined with partition-dependent variable undersampling (8-24 rays/partition) for 188ms shot duration, timed to end-systole.
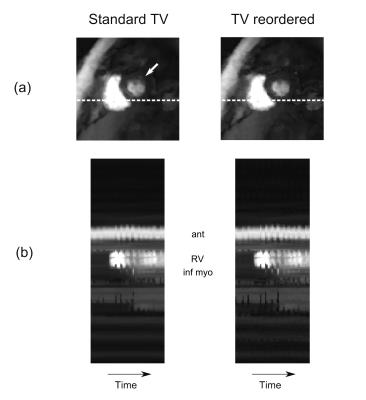
Reconstruction of the ~15x undersampled datasets used a spatially and temporally constrained total variation CS algorithm5 adapted to 3D (αtemporal = 3x10-5, αin-plane = 3x10-4, αthrough-plane = 1x10-5, Niterations = 250). The temporal constraint underwent temporal pixel-wise reordering prior to each iteration, using an initial reconstruction as a prior, improving its robustness to motion by better fitting the signal to the constraint6. The reconstructions with and without this extra step were compared.
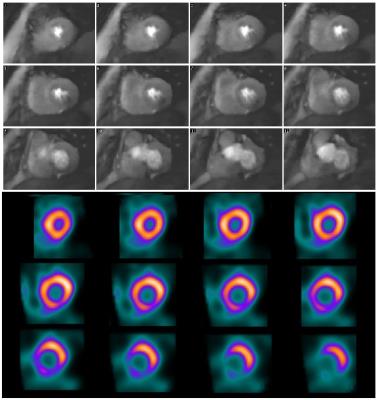
Comparison was made with myocardial perfusion scintigraphy (MPS) in a patient with known CAD who had recently (<6 weeks) undergone a stress Thallium protocol on a solid state SPECT camera.
Results
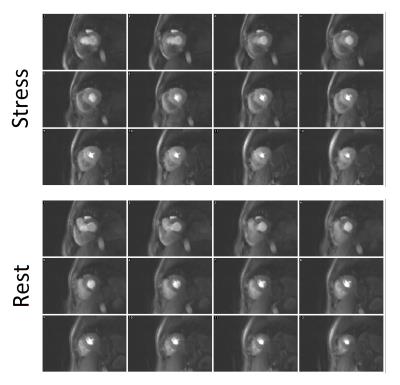
All 12 subjects were successfully scanned (Figure 1) tolerating a wide range of respiratory motions (e.g. Figure 2) in rest and stress, reaching heart rates up to 97bpm.
Despite this, degradation of the image quality during the first-pass by respiratory motion was rarely obvious. Application of the reordering scheme during reconstruction was able to improve the image quality and temporal dynamics of the datasets when minor respiratory effects were observed (Figure 2).
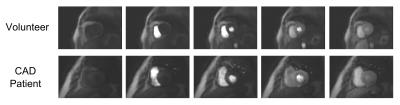
Subjects with no suspected CAD were clear of any persistent hypointense myocardial regions (Figure 3), whilst the patient with known CAD showed an apparent inferior/infero-lateral defect in the basal to midventricular images, matching well with the MPS scan (Figure 4).
Demonstration in greater numbers with known or suspected coronary artery disease is still necessary, for which data is currently being acquired.
Conclusions
The technical reliability suited to routine clinical use of 3D FPP at stress and rest during free-breathing was demonstrated. This included the first known detection and confirmation of a perfusion defect in CS reconstructed 3D FPP.
The pixel reordering method for the reconstruction algorithm adds further potential to maintain fidelity during respiratory motion, although the reconstructions appeared robust in all acquired datasets despite the large range of respiratory motions.
Acknowledgements
This work was supported by the NIHR Cardiovascular Biomedical Research Unit of Royal Brompton and Harefield NHS Foundation Trust and Imperial College London, UK.
MJF is funded by a British Heart Foundation (BHF) PhD Studentship Grant - FS/13/21/30143.
References
1. Fair MJ, Gatehouse, PD, DiBella EVR, et al. A review of 3D first-pass, whole-heart, myocardial perfusion cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2015;17(1):68.
2. Jogiya R, Morton G, Silva K, et al. Ischemic burden by three-dimensional myocardial perfusion cardiovascular magnetic resonance: comparison with myocardial perfusion scintigraphy. Circ Cardiovasc Imaging. 2014;7(4):647-54.
3. Manka R, Wissmann L, Gebker R, et al. Multicenter evaluation of dynamic three-dimensional magnetic resonance myocardial perfusion imaging for the detection of coronary artery disease defined by fractional flow reserve. Circ Cardiovasc Imaging. 2015;8(5).
4. Roeloffs V, Voit D and Frahm, J. Spoiling without additional gradients: radial FLASH MRI with randomized radiofrequency phases. Magn Reson Med. 2015;75(5):2094-9.
5. Adluru G, MCGann C, Speier P, et al. Acquisition and reconstruction of undersampled radial data for myocardial perfusion magnetic resonance imaging. J Magn Reson Imaging. 2009;29(2):466-73.
6. Adluru G and DiBella EV, Int J Biomed Imaging. Reordering for improved constrained reconstruction from undersampled k-space data. 2008;341684 doi: 10./1109/ISBI.2007.356800.
Figures