0521
Fast multi-slice B1 and B0 mapping (B01TIAMO) for 32-channel pTx body MRI at 7 Tesla1Erwin L. Hahn Institute for Magnetic Resonance Imaging, University Duisburg-Essen, Essen, Germany, 2High Field and Hybrid MR Imaging, University Hospital Essen, Essen, Germany, 3Donders Institute for Brain, Cognition and Behaviour, Radboud University Nijmegen, Nijmegen, Netherlands, 4Medical Physics in Radiology, German Cancer Research Center (DKFZ), Heidelberg, Germany
Synopsis
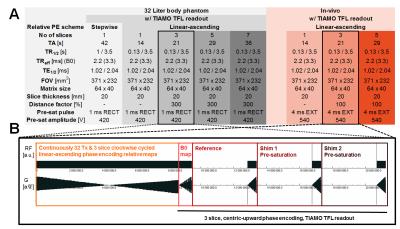
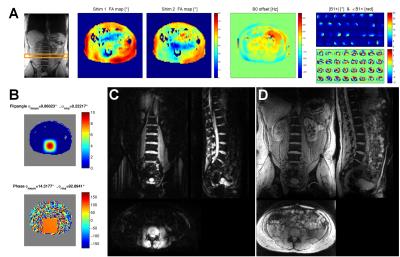
The aim of this work was to improve the recently developed B1TIAMO method at ultra-high magnetic field for a 32-channel body transceiver array by providing complete information about both the B0 and B1+ distribution within the human abdomen without movement artifacts by breathing. Therefore, a fast multi-slice version including two-echo B0 maps with time interleaved acquisition of modes (B01TIAMO) was introduced. Furthermore, B1+ phase calculation was improved by geometric-decomposition coil compression, resulting in accurate single-channel B1+ and B0 maps for three different slices within only 21s versus the 42s step-by-step measurement for a single slice with normal B1TIAMO.
Purpose
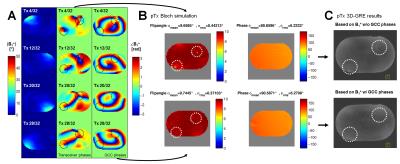
Parallel transmit (pTx) body imaging1,2 requires accurate knowledge of the individual transmit channel magnitude/phase distribution (B1+) over a large field-of-view supplemented by the local frequency offset of B0. For non-moving body regions, these datasets are often acquired over several minutes, which is not feasible for the human torso due to breathing and organ motion. Furthermore, B1+ mapping at ultra-high magnetic field is limited for such a large volume because a high dynamic flip angle (FA) range is necessary for absolute scaling. Hence, low local signal-to-noise ratios (SNR) additionally result in phase singularities due to normalization onto one transceiver (Tx/Rx) channel. Therefore, in this work the B1TIAMO technique3 for fast and accurate B1+ mapping of a 32-channel Tx/Rx array was expanded not only by the geometric-decomposition coil compression (GCC)4 post-processing, but a much faster joint multi-slice measurement together with time interleaved acquisition of modes5 B0 mapping was also included. Methods
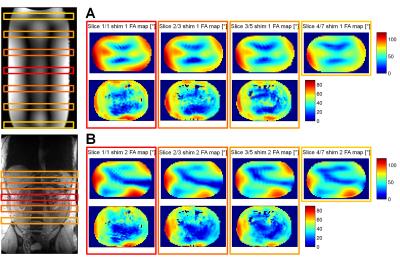
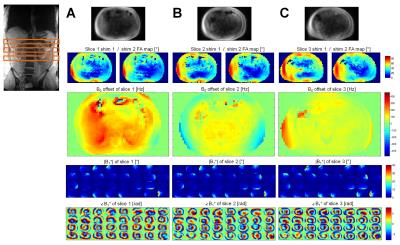
Results
Discussion and Conclusion
The overall B1+ data quality of the B1TIAMO technique could be improved by the GCC method. Moreover, for the purpose of pTx applications, the additional information from B0 mapping without significant total measurement time prolongation is recommended. For multi-slice B01TIAMO, the absolute FA map signal loss of 10-20%, depending on T1 time, has to be considered; hence, splitting up the acquisition into more pre-saturation blocks should be investigated to ameliorate the signal loss. However, this effect might be outweighed by the significant time gain for in-vivo B0/B1+ mapping. As an outlook, B0/B1+ information in three orthogonal directions over a large field-of-view would facilitate elaborate 3D abdominal pTx.
Acknowledgements
The research leading to these results has received funding from the European Research Council under the European Union's Seventh Framework Programme (FP/2007-2013) / ERC Grant Agreement n. 291903 MRexcite.References
[1] Katscher U, Boernert P, Leussler C, et al. Transmit SENSE. (2003) MRM 49(1): 144-50.
[2] Grissom W, Yip CY, Zhang Z, et al. Spatial domain method for the design of RF pulses in multicoil parallel excitation. (2006) MRM 56(3): 620-9.
[3] Brunheim S, Orzada S, Gratz M et al. Combined B1 mapping with TIAMO for fast and accurate multi-channel RF shimming in 7 Tesla body MRI. (2016) Proc. Intl. Soc. Mag. Reson. Med. 16: p. 936.
[4] Zhang T, Pauly JM, Vasanawala SS, Lustig M. Coil compression for accelerated imaging with Cartesian sampling. (2013) MRM 69(2): 571-582.
[5] Orzada S, Maderwald S, Poser BA et al. RF excitation using time interleaved acquisition of modes (TIAMO) to address B1 inhomogeneity in high-field MRI. (2010) MRM 64(2): 327-333.
[6] Orzada S, Bitz AK, Kraff O et al. A 32-channel integrated body coil for 7 Tesla whole-body imaging. (2016) Proc. Intl. Soc. Mag. Reson. Med. 24: p. 167.
[7] Shooshtary S, Gratz M, Ladd ME, Solbach K. High-Speed RF Modulation System for 32 Parallel Transmission Channels at 7T. (2014) Proc. Intl. Soc. Mag. Reson. Med. 22: p. 544.
[8] Orzada S, Bitz AK, Solbach K, Ladd ME. A receive chain add-on for implementation of a 32-channel integrated Tx/Rx body coil and use of local receive arrays at 7 Tesla. (2015) Proc. Intl. Soc. Mag. Reson. Med. 23: p. 3134.
[9] Van de Moortele PF, Snyder C, DelaBarre et al. Calibration tools for RF shim at very high field with multiple element RF coils: From ultra fast local relative phase to absolute magnitude B1+ mapping. (2007) Proc. Intl. Soc. Mag. Reson. Med. 15: p. 1676.
[10] Robinson S, Grabner G, Witoszynskyj S, Trattnig S. Combining phase images from multi-channel RF coils using 3D phase offset maps derived from a dual-echo scan. (2011) MRM 65(6): 1638–1648.
Figures