0520
Simultaneous Multi-Contrast Imaging with Readout-Segmented EPI1MR Physics, Fraunhofer MEVIS, Bremen, Germany, 2Athinoula A. Martinos Center for Biomedical Imaging, MA, United States, 3mediri GmbH, Heidelberg, Germany
Synopsis
A new method is presented for acquiring multiple image contrasts simultaneously. The technique reduces patient examination times and facilitates accurate image registration between contrasts. This work focuses on a variant of the method, in which readout-segmented EPI (rs-EPI) is used to perform high-quality, navigator-corrected, diffusion-weighted imaging simultaneously with a T2*-weighted acquisition. This combination of contrasts has clinical significance in acute stroke, providing a registered data set for assessing the infarct and possibility of associated hemorrhage. The proposed method modifies the contrast as a function of slice position and uses blipped CAIPIRINHA and slice-GRAPPA to separate the contrasts into individual images.
Purpose
Clinical MRI examinations acquire data with multiple image contrasts as a succession of independent measurements. The scan time of the individual measurements can be reduced using simultaneous multi-slice (SMS) imaging1,2, but this is only effective for protocols with long repetition times (TR) that can be reduced without significant effect on image contrast or SNR. However, many clinical protocols use TR values that cannot be shortened without compromising image quality. This paper introduces an alternative approach, simultaneous multi-contrast (SMC) imaging, in which SMS is adapted to acquire signals simultaneously with multiple contrasts within a single measurement. As an initial example, the current work explores the simultaneous acquisition of diffusion-weighted (DW) and T2*-weighted images using a modified readout-segmented EPI (rs-EPI) sequence3. This combination of contrasts has particular clinical relevance for acute stroke, where examination time is a critical factor.
Methods
Pulse Sequence:
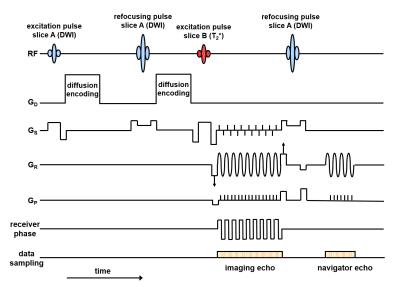
In the rs-EPI sequence for SMC (Fig. 1), data are acquired from two slice positions at the same time with one slice (A) generating DW while the other slice (B) provides T2*-weighted contrast. Firstly, slice A is excited and DW preparation is applied. Then slice B is excited before both signals are sampled simultaneously using rs-EPI with a variable amplitude encoding gradient in the readout (GR) direction (labelled with arrows in Fig. 1) and a blipped phase-encoding gradient (GP). A blipped-CAIPIRINHA4,5 gradient scheme along the slice-select (GS) direction is used in conjunction with receiver phase modulation to shift the T2*-weighted image by half a field of view (FOV) in the phase-encoding direction relative to the DW image. Finally, an RF refocusing pulse is applied to slice A only to generate a 2D navigator signal to phase correct the DW imaging data.
Data Acquisition:
Data were acquired from a healthy subject at 3T (MAGNETOM Skyra, Siemens Healthcare GmbH) with the following parameters: (both contrasts) FOV 200mm, matrix 192x192, slice thickness 4mm, TR 4000ms; (DW contrast) TE 118ms, b-values zero and 1000 s/mm2; (T2*-weighted contrast) TE 40ms, flip angle 45°. Separate multi-slice reference data were acquired for DW contrast with b=0 and for T2*-weighted contrast; in both cases, data were acquired using a single-shot scan corresponding to the central readout segment.
Image Processing:
The slice-GRAPPA reconstruction5 with inter-slice leakage artefact reduction6 was used to separate the aliased slices into single-slice data. The reference data were used to fit separate 3 x 3 kernels, or weight sets. The same weight sets were used at all b-values to separate DW and T2*-weighted images. The reconstruction algorithm was implemented using the manufacturer’s proprietary reconstruction software in a way that enables online reconstruction.
Results
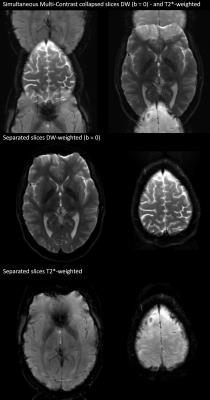
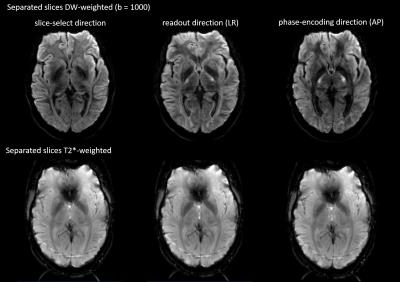
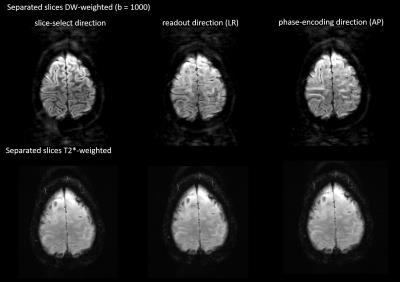
The top row of Fig. 2 shows the result of the simultaneous acquisition of DW data with a b=0 and T2*-weighted data from a separate slice position. The DW image is at the center of the FOV and the T2*-weighted image is aliased to the edge of the FOV. The separated contrasts are displayed in the second and third rows of the figure. Figs. 3 and 4 show the result of applying the slice-GRAPPA procedure to the SMC data with combined T2* contrast and DW contrast with b=1000s/mm2.Discussion
The separated images in Figs. 2, 3, and 4 demonstrate the feasibility of acquiring two MRI contrasts simultaneously and separating the aliased slices using the slice-GRAPPA approach. The DW images with b = 1000s/mm2 shows some residual signal in the background of the second slice (Fig. 4). This emphasizes the need for further optimization of the slice-GRAPPA algorithm for SMC. The T2*-weighted images also show artefacts resulting from an unsuppressed fat signal because the current method of fat suppression in the sequence is specific to the DW contrast (slice-select gradient reversal). A further limitation to the current study is that in-plane parallel imaging was not used; this greatly improves the image quality in rs-EPI by reducing the TE and the effective echo spacing and will be explored in future work, along with the possibility of acquiring more than two contrasts simultaneously.
Conclusion
This work has introduced a new class of MRI acquisition in which a slice-dependent image contrast allows SMS imaging techniques to simultaneously acquire MR images at multiple contrasts. The technique is likely to improve scanning efficiency in a variety of clinical situations and to reduce image registration issues when subjects move between measurements. The specific SMC sequence used in this study is of direct relevance to clinical examinations in acute stroke7, providing an additional T2* weighted image contrast without compromising the image quality of the DW rs-EPI images or significantly extending the scan time.Acknowledgements
All funding for this study was provided by the internal Attract funding program of the German Fraunhofer-Gesellschaft.References
1. Larkman DJ, Hajnal JV, Herlihy AH, Coutts GA, Young IR, Ehnholm G. Use of multicoil arrays for separation of signal from multiple slices simultaneously excited. J Magn Reson Imaging 2001;13:313-317.
2. Moeller S, Yacoub E, Olman CA, Auerbach E, Strupp J, Harel N, Ugurbil K. Multiband multislice GE-EPI at 7 tesla, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain fMRI. Magn Reson Med 2010;63:1144-1153.
3. Frost R, Jezzard P, Douaud G, Clare S, Porter DA, Miller KL. Scan
Time Reduction for Readout-Segmented EPI Using Simultaneous Multislice
Acceleration: Diffusion-Weighted Imaging at 3 and 7 Tesla. Magn Reson Med
2015;74:136-149.
4. Breuer FA, Blaimer M, Heidemann RM, Mueller MF, Griswold MA, Jakob PM. Controlled Aliasing in Parallel Imaging Results in Higher Acceleration (CAIPIRINHA) for Multi-Slice Imaging. Magn Reson Med 2005;53:684-691.
5. Setsompop K, Gagoski BA, Polimeni JR, Witzel T, Wedeen VJ, Wald LL. Blipped-controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g-factor penalty. Magn Reson Med 2012;67:1210-1224.
6. Cauley SF, Polimeni JR, Bhat H, Wald LL, Setsompop K. Interslice leakage artifact reduction technique for simultaneous multislice acquisitions. 2014;72(1):93-102.
7. Schellinger PD, Jansen O, Fiebach JB, Hacke W0, Sartor K. A Standardized MRI Stroke Protocol Comparison with CT in Hyperacute Intracerebral Hemorrhage. Stroke 1999;30:765-768.
Figures