0514
Atypical maturation of short-range fibers connecting higher-order brain regions in children with autism aged 2-7 yearsMinhui Ouyang1, Jennifer Muller1, Hua Cheng2, Yun Peng2, J. Christopher Edgar1,3, Timothy P.L. Roberts1,3, and Hao Huang1,3
1Radiology, Children's Hospital of Philadelphia, Philadelphia, PA, United States, 2Radiology, Beijing Children's Hospital, Capital Medical University, Beijing, People's Republic of China, 3Radiology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, United States
Synopsis
A pattern of local or short-distance “over-connectivity” and long-range under-connectivity is frequently hypothesized in individuals with autism spectrum disorder (ASD). Little is known about the spatiotemporal characterization of structural short-distance connections in typically developing (TD) children or children with ASD. We hypothesized that altered trajectories of short-range association fibers (SAF) are not uniform across the brain regions, with abnormal maturation primarily observed in higher-order but not in primary sensory brain regions in children in ASD. Here, we quantified SAF with a novel index defined as normalized SAF (NSAF) based on diffusion MRI tractography, and characterized its trajectories across brain regions.
Purpose
The pattern of local or short-distance “functional over-connectivity”, in parallel to the long-range under-connectivity, has been frequently suggested in individuals with autism spectrum disorder (ASD) [1-6]. Both functional and structural long-distance connectivity appears to be weaker in ASD than in controls [e.g. 7-8]. However, little is known about spatiotemporal characterization of “structural” short-distance connections in typically developing (TD) children or children with ASD. We hypothesized that altered trajectories of short-range association fibers (SAF) are not uniform across brain regions, and altered trajectories occur in higher-order brain regions in children in ASD. We quantified SAF across the brain regions with a novel index defined as normalized SAF (NSAF), defined as the ratio of the number of SAF to the number of all cortico-cortical connectivity fibers (sum of SAF and long-range association fibers, LAF) traced from a given gyrus with diffusion MRI (dMRI) tractography. The goal is to delineate the cortical gyri whose age-dependent short-range connections undergo altered trajectories in children with ASD aged 2-7 years versus age-matched children with TD. The delineated over-connectivity may provide structural basis for the functional over-connectivity often reported in ASD [1-3].Methods
Participants: 31 children with ASD aged 2-7 years and 20 age-matched TD children participated. Acquisition of dMRI and T1-weighted image: All MR scans were performed on a 3T Philips Achieva MR system. dMRI were acquired using single-shot EPI with SENSE=2.3. Other parameters were: TR/TE=7960/83ms, FOV=256x256mm2, imaging resolution=2x2x2mm3, 70 slices, 30 independent diffusion-weighted directions, b-value=1000sec/mm2. T1-weighted images were acquired using MPRAGE sequence with imaging resolution=1x1x1mm3. Fiber tracing from a parcellated cortical gyrus: Using the T1-weighted image, the brain cortical surface was rendered and parcellated into 68 gyral labels [9] using Freesurfer(http://surfer.nmr.mgh.harvard.edu). Fiber assignment of continuous tractography (FACT) [10] was used to trace the whole brain fibers for all subjects in DiffusionToolkit(http://www.trackvis.org/dtk/) with an angular threshold of 60o. Using inferior parietal gyrus(IPG) as an example, the parcellated cortical ribbon transformed from T1-weighted image space(Fig. 1a) to dMRI space(Fig. 1b) was then dilated by 8 mm (in green, Fig. 1b) with in-house program to get through the dense white matter zone [11]. All association fibers traced from IPG are shown in Fig. 1c. Categorization of long- and short-range fibers based on termination location of the other end of fibers: The adjacent and non-adjacent gyral labels of each cortical gyrus were identified. Using IPG (shown in green, Fig. 1d) as an example, its adjacent gyri are SPG(yellow), LOG(red) and SMG(blue). All other gyri are non-adjacent gyri for IPG. Association fibers initiated from IPG can then be categorized into short- and long-range based on the other end of the fibers terminating in adjacent and non-adjacent gyri to IPG, respectively(Fig. 1e). Developmental curves of regional NSAF in both ASD and TD groups: Regional NSAF of a certain gyrus was calculated as the ratio of the number of SAF to total number of cortico-cortical fibers (sum of SAF and LAF) connecting to this gyrus. To investigate the developmental curve of regional NSAF, linear regression was performed between NSAF values and age in four representative functional regions: prefrontal cortex, default-mode network (DMN) hub (precuneus cortex), primary somatosensory (S1) and primary visual (V1) cortex.Results
Fig. 2 demonstrates the developmental curves of regional NSAF from two representative higher-order functional regions in TD and ASD. The three-dimensionally reconstructed LAF (green) and SAF (red) connected to the cortical region are also shown on the top of Fig. 2. In higher-order functional regions, in the TD group the NSAF value decreased significantly from 2 to 7 years in both prefrontal cortex (p=0.05, Fig. 2a) and DMN-hub (p=0.04, Fig. 2b). In contrasts, in ASD the NSAF values from these two regions were not significantly associated with age (p>0.05, Fig. 2), indicating atypical maturation of short-range connectivity. On the other hand, in the TD group the NSAF from primary sensory regions such as V1 and S1 were not significantly associated with age (p>0.05). Similarly, in ASD, associations between regional NSAF of S1 or V1 and age were also not observed.Discussion and conclusion
The proposed NSAF metric revealed that the age-dependent trajectories of short-range connections in children with ASD are altered in higher-order but not in primary sensory brain regions. The non-uniform alterations indicate that higher-order brain regions are of specific interest in children with ASD aged 2-7 years. Gradual decreases of NSAF in higher-order brain regions were found in normal brain development. The findings from this study may offer structural basis for the functional “over-connectivity” described in ASD [1-3]. Analysis of functional short-range connectivity with functional MRI data is under way.Acknowledgements
This study is funded by NIH MH092535, MH092535-S1, HD086984 and MH107506.References
[1] Rudie et al (2013) Cell Reports 5: 565. [2] Keown et al (2013) Cell Reports 5:567. [3] Supekar et al (2013) Cell Reports 5:738. [4] Courchesne et al (2007) Neuron 56:399. [5] Wass (2011) Brain Cogn 75:18. [6] Vissers et al (2012) Neurosci Biobehav Rev 36:604. [7] Courchesne and Pierce (2005) Curr Opin Neurobiol 15:225. [8] Martino et al. (2014) Mol. Psychiatry 19:659. [9] Desikan et al (2006) Neuroimage 31:968. [10] Mori et al (1999) Ann Neurol 45:265. [11] Reveley et al (2015) Proc Natl Acad Sci USA 112:E280Figures
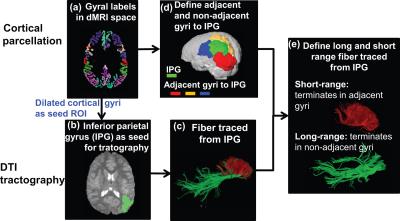
Fig. 1: The schematic
pipeline of cortical parcellation (a), fiber tracing (b,c) and categorization
of long- and short- range fibers (d, e) from a certain cortical gyrus. In d,
IPG shown in green, superior parietal gyrus
(SPG, in yellow), lateral occipital gyrus (LOC, in red) and supra marginal
gyrus (SMG, in blue).
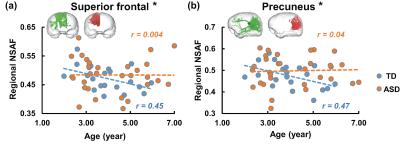
Fig. 2: Developmental curve
of regional NSAF from two higher-order functional regions: prefrontal cortex (a)
and default-mode network hub (b). Each circle in the scatter plot represents
the NSAF from one child with TD (blue) or ASD (orange). The dashed lines (blue for TD and orange for ASD) were linearly fitted
from regional NSAF value. R values
(blue for TD and orange for ASD) are correlation coefficients of regional NSAF
and age.