0512
Real-time fMRI Neurofeedback of the Amygdala Enhances Amygdala-orbitofrontal Connectivity and Lateralized EEG Coherence in Veterans with Combat-related PTSD1Laureate Institute for Brain Research, Tulsa, OK, United States, 2Laureate Psychiatric Clinic and Hospital, Tulsa, OK, United States, 3Neuroscience Dept., George Mason University, Fairfax, VA, United States, 4Dept. of Psychological Science, University of Arkansas, Fayetteville, AR, United States, 5College of Engineering, Stephenson School of Biomedical Engineering, University of Oklahoma, Tulsa, OK, United States
Synopsis
We have performed a study of emotion regulation training in veterans with combat-related PTSD using real-time fMRI neurofeedback (rtfMRI-nf) with simultaneous EEG. Eighteen PTSD patients learned to upregulate their left amygdala activity using rtfMRI-nf during a positive emotion induction task based on retrieval of happy autobiographical memories. Enhancement in the amygdala-orbitofrontal functional connectivity during the rtfMRI-nf task showed positive correlation with severity of PTSD symptoms. Enhancement in left-lateralized upper alpha EEG coherence also positively correlated with PTSD severity. These results suggest that the rtfMRI-nf of the amygdala has the potential to correct the functional connectivity deficiencies specific to PTSD.
Purpose
Real-time fMRI neurofeedback (rtfMRI-nf) of the amygdala with simultaneous EEG1 allows modulation of the amygdala network activity and investigation of related EEG effects. Here we examine changes in fMRI functional connectivity and EEG coherence during rtfMRI-nf training of the left amygdala (LA) in veterans with combat-related PTSD, performing a positive emotion induction task based on retrieval of happy autobiographical memories. Our results show enhancement in the LA functional connectivity with the lateral orbitofrontal cortex (LOFC, BA 47) and medial orbitofrontal cortex (MOFC, BA 11), which play important roles in emotion regulation2, motivation3, decision-making4, social cognition, and moral judgment5. Furthermore, the enhancement in the LA functional connectivity with the LOFC and MOFC positively correlates with severity of PTSD symptoms. Left-lateralized enhancement in upper alpha EEG coherence exhibits a similar positive correlation with PTSD severity.Methods
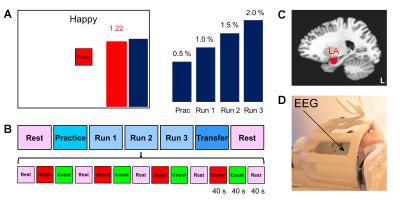
Twenty male patients with a primary diagnosis of PTSD related to combat trauma participated in the study and completed the first training session (Fig. 1) involving rtfMRI-nf with simultaneous EEG. Clinical assessment included the Clinician-Administered PTSD Scale for DSM-IV (CAPS), the Hamilton Depression Rating Scale (HDRS), and other instruments. Data from 18 patients were included in the group analyses.
The experiments were performed on a GE Discovery MR750 3T MRI scanner with an 8-channel receive-only head coil. A gradient echo EPI sequence with FOV/slice=240/2.9 mm, TR/TE=2000/30 ms, SENSE R=2, image matrix 96×96, flip=90°, 34 axial slices, was employed for fMRI. Simultaneous EEG recordings were performed using a 32-channel MR-compatible EEG system (Brain Products GmbH) in 0.016−250 Hz band with 0.1 µV resolution and 5 kS/s sampling. The rtfMRI-nf was implemented using a custom real-time system with a neurofeedback GUI (Fig. 1A)1. The nf signal was based on fMRI activation in the LA target ROI (Fig. 1C)1. The rtfMRI-nf session protocol (Fig. 1B) included seven runs, and each run (except Rest) consisted of 40-s blocks of Rest, Happy Memories, and Count conditions. For each Happy Memories condition, the participant was asked to feel happy by evoking happy autobiographical memories, while trying to raise the level of the red bar to that of the blue target bar (Fig. 1A). The height of the target bar was increased linearly from run to run (Fig. 1A) to introduce a linear trend across the four rtfMRI-nf runs1.
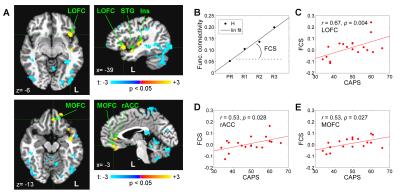
fMRI data analysis was performed in AFNI6. GLM functional connectivity analysis was conducted for Happy Memories conditions in each run, using the LA target ROI (Fig. 1C) as the seed. The LA connectivity maps for the four rtfMRI-nf runs were concatenated. Linear trend across the runs was evaluated using the 3dTfitter AFNI program, and the functional connectivity slope (FCS) was determined for each voxel (Fig. 2B). Group analysis was performed on the FCS data using the 3dttest++ AFNI program with the patients’ CAPS and HDRS ratings included as two covariates.
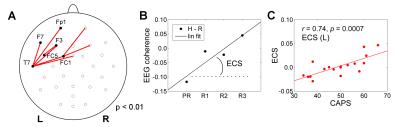
EEG data analysis was conducted in BrainVision Analyzer 2. Artifacts were removed using average artifact subtraction and ICA. Upper alpha EEG band was defined as [IAF…IAF+2] Hz, where IAF is the individual alpha peak frequency1. EEG coherence was computed for each pair of channels as the ratio of cross-spectrum and auto-spectrum, separately for Happy Memories and Rest conditions in each run1. The EEG coherence slope (ECS) for the upper alpha band was determined for each channel pair (Fig. 3B).
Results
The FCS showed significant positive correlations with CAPS (controlled for HDRS) for the left LOFC, MOFC, rACC, and some other regions (Fig. 2A). These effects are further illustrated in Figs. 2C-E. Similarly, the ECS exhibited significant positive correlations with CAPS (controlled for HDRS) for several pairs of fronto-temporal EEG channels with left lateralization (Fig. 3A). This effect is illustrated in Fig. 3C.Discussion
During recollection of emotional memories, PTSD patients generally exhibit reduced BOLD activity in the rACC, MOFC, LOFC and other brain regions7. Our results in Fig. 2 show the enhancement in the LA functional connectivity with these regions, which is stronger in patients with more severe PTSD (higher CAPS). This positive correlation suggests that the rtfMRI-nf of the amygdala has the potential to correct (reverse) the functional connectivity impairments specific to PTSD, in agreement with other studies8. The results in Fig. 3 demonstrate the positive correlation between the enhancement in upper alpha EEG coherence over the left fronto-temporal areas and PTSD severity. This observation is consistent with similar findings in depression1. It suggests that the rtfMRI-nf has the potential to correct the approach motivation deficiencies specific to PTSD.Acknowledgements
This research was supported by W81XWH-12-1-0697 grant from the US Department of Defense.References
1. Zotev V, Yuan H, Misaki M, et al. Correlation between amygdala BOLD activity and frontal EEG asymmetry during real-time fMRI neurofeedback training in patients with depression. NeuroImage Clin. 2016; 11:224-238.
2. Ochsner KN, Bunge SA, Gross JJ, et al. Rethinking feelings: an fMRI study of the cognitive regulation of emotion. J Cogn Neurosci. 2002; 14:1215-1229.
3. Spielberg JM, Miller GA, Warren SL, et al. A brain network instantiating approach and avoidance motivation. Psychophysiol. 2012; 49:1200-1214.
4. Rushworth MFS, Noonan MP, Boorman ED, et al. Frontal cortex and reward-guided learning and decision-making. Neuron 2011; 70:1054-1069.
5. Forbes CE, Grafman J. The role of the human prefrontal cortex in social cognition and moral judgment. Annu Rev Neurosci 2010; 33:299-324.
6. Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 1996; 29:162-173.
7. Lanius RA, Williamson PC, Hopper J, et al. Recall of emotional states in posttraumatic stress disorder: an fMRI investigation. Biol Psychiatry 2003; 53:204-210.
8. Nicholson AA, Rabellino D, Densmore M, et al. The neurobiology of emotion regulation in posttraumatic stress disorder: amygdala downregulation via real-time fMRI neurofeedback. Hum Brain Mapp. 2016; in press.
Figures