0511
Investigating Brain Connectomic Alterations in PTSD and PCS using the Reproducibility of Independent Components obtained from Resting-State Functional MRI Data1Department of Computer Science and Software Engineering, Auburn University, Auburn, AL, United States, 2AU MRI Research Center, Department of Electrical and Computer Engineering, Auburn University, Auburn, AL, United States, 3Department of Psychiatry and Biobehavioral Sciences, University of California Los Angeles, Los Angeles, CA, United States, 4Human Dimension Division, HQ TRADOC, Fort Eustis, Fort Eustis, VA, United States, 5U.S. Army Aeromedical Research Laboratory, Fort Rucker, Fort Rucker, AL, United States, 6Department of Psychology, Auburn University, Auburn, AL, United States, 7Alabama Advanced Imaging Consortium, Auburn University and University of Alabama Birmingham, Birmingham, AL, United States
Synopsis
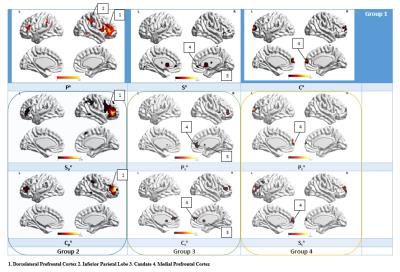
Posttraumatic stress disorder (PTSD) and Post-concussion syndrome (PCS) are heterogeneous neurological disorders where fMRI connectivity metrics derived from them may not be highly reproducible, leading to poor generalizability and consequently lower classification accuracies. We present a method that characterizes the reproducibility of networks using ‘generalized Ranking and Averaging Independent Component Analysis by Reproducibility’ (gRAICAR) algorithm followed by unsupervised clustering to discriminate between the groups based on functional brain networks that are most reproducible within PTSD, PCS, and healthy control groups separately. We identify dorsolateral prefrontal cortex, inferior parietal lobule, caudate and medial prefrontal cortex as regions within the most reproducible independent components.
Purpose
Posttraumatic Stress Disorder (PTSD) symptoms can either emerge soon after experiencing a traumatic event or years after the event, thus leading to its classification as a heterogeneous disorder not only from the standpoint of the occurrence following a trauma but also from that of symptoms experienced1. Mild traumatic brain injury (mTBI) presents a broad range of clinical features indicating that its underlying pathologic features are highly heterogeneous2. Military service members sustaining mTBI are at the risk of developing prolonged symptoms or post-concussion syndrome (PCS). Even though PTSD can be characterized by functional hyper-connectivity3,4, making such a determination in PCS has led to mixed or inconclusive results5. We hypothesized that functional brain networks that are most reproducible in PTSD/PCS and healthy control groups separately may possess the ability to distinguish effectively between the groups. The proposed method is based upon the reproducibility of independent components obtained from resting-state fMRI, followed by the application of unsupervised learning techniques and analysis of these components to evaluate their ability in discriminating between the groups6.Methods
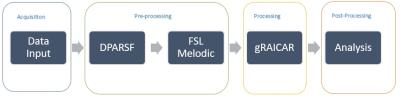
Resting-state fMRI data of 71 soldiers having combat experience in Iraq and/or Afghanistan were acquired in a Siemens 3T Verio scanner, with TR/TE=600/30ms, voxel-size=3×3×5mm3, 1000 volumes and 2 sessions7. This dataset included 15 subjects with PTSD, 30 with both PCS and PTSD (PCS+PTSD), and 26 matched combat controls. We first pre-processed these data including realignment, normalization to MNI space, spatial smoothing (8mm kernel), de-trending and temporal band-pass filtering [0.01, 0.1Hz] using DPARSF toolbox with SPM8, followed by the application of the MELODIC algorithm9 in FSL10 to obtain independent components (ICs) at both the individual subject and group levels. These were input into the ‘generalized Ranking and Averaging Independent Component Analysis by Reproducibility’ (gRAICAR) algorithm11 in order to retrieve the most reproducible group-level components from the PTSD, PCS+PTSD and the control groups. Components selected based upon inter-subject consistency for the group-level component within PTSD (127), PCS+PTSD (82), and matched control (111) groups were examined further in post-gRAICAR processing (see Fig.1 for workflow). We obtained individual subject spatial maps of each group-level component from all three groups and stacked them into matrices P (PTSD), S (PCS+PTSD), and C (Control). We then performed k-means clustering after pairing P, S, and C for group-level component permutations in order to examine whether individual subject ICs cluster into (unsupervised) PTSD, PCS+PTSD and control clusters. Components from PTSD (Px), PCS+PTSD (Sx) and control (Cx) groups that formed the purest clusters, i.e. with the highest accuracy for discrimination when combined, were then examined further. We identified components called Spx and Cpx in the PCS+PTSD and control groups respectively, which had the highest spatial correlation with Px, components Psx and Csx in the PTSD and control groups respectively which had the highest spatial correlation with Sx, and finally components Pcx and Scx in the PTSD and PCS+PTSD groups respectively which had the highest spatial correlation with Cx. We performed another clustering analysis, on Px paired with Spx and Cpx, on Sx paired with Psx and Csx, and finally on Cx paired with Pcx and Scx. The second round of analysis determined whether the reproducible components in each group, when paired with the corresponding components with similar spatial distribution in the other groups, effectively discriminated between the groups.Results
Fig.1. shows the spatial maps for the most reproducible ICs in each of the groups. The highest classification accuracy values were obtained by pairing Px, Sx, and Cx. All the three groups (PTSD, PCS+PTSD, control) were identified with 100% accuracy using this combination. Px, paired with Spx and Cpx produced classification accuracies of 93% for PTSD, 83.3% for PCS+PTSD, and 84.6% for the control group. Sx when paired with Psx and Csx produced classification accuracies of 100% for PTSD, 96.7% for PCS+PTSD, and 100% for the control groups. Cx when paired with Pcx and Scx produced classification accuracies of 86.7% for PTSD, 96.7% for PCS+PTSD, and 92.3% for the control groups.Discussion
Poor generalizability and lower classification accuracies are the norm in neuroimaging-based classification of psychiatric disorders like PTSD and PCS. We presented a novel method for the characterization of reproducibility of networks using gRAICAR algorithm, followed by unsupervised clustering to discriminate between the groups. We identified functional brain networks that are most reproducible within PTSD, comorbid PCS+PTSD and control groups separately, which yielded high classification accuracies. Our results demonstrate that functional brain networks that are most reproducible within PTSD, PCS and matched control groups separately possess the ability to distinguish effectively between these groups.Acknowledgements
We thank NSF (grant # 0966278) for funding the data analysis part of this study. The authors acknowledge financial support for data acquisiion from the U.S. Army Medical Research and Materiel Command (MRMC) (Grant # 00007218). The views, opinions, and/or findings contained in this article are those of the authors and should not be interpreted as representing the official views or policies, either expressed or implied, of the U.S. Army or the Department of Defense (DoD). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors thank the personnel at the TBI clinic and behavioral health clinic, Fort Benning, GA, USA and the US Army Aeromedical Research Laboratory, Fort Rucker, AL, USA, and most of all, the soldiers who participated in the study. The authors thank Julie Rodiek and Wayne Duggan for facilitating data acquisition.References
1. Sartory et al. PLoS ONE 8(3): e58150. 2. Rosenbaum et al., Brain Imaging and Behavior. 2012;6: 255. 3. Larson et al., Rehab Psych. 2013; 8(3):245-52. 4. Hayes et al., Front Integ Neurosci. 2012; 9;6:89. 5. Eierud et. al., Neuroimage Clin. 2014; 4:283-94. 6. Syed et al., Proc. Intl. Soc. Mag. Reson. Med. 2015; 23, 2015. 7. Rangaprakash et al., Proc. Intl. Soc. Mag. Reson. Med. 2015; 23, 2015. 8. www.restfmri.net/forum/DPARSF 9. http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/MELODIC 10. Jenkinson et al., Neuroimage, 62(2):780-90, 2012. 11. Yang et al., Neuroimage, 63(1):403-414, 2012. 12. Xia et al., PLoS ONE 8:e68910Figures