0510
Effective Connectivity Network of Emotion Regulation in Soldiers with Trauma1AU MRI Research Center, Department of Electrical and Computer Engineering, Auburn University, Auburn, AL, United States, 2Department of Psychiatry and Biobehavioral Sciences, University of California Los Angeles, Los Angeles, CA, United States, 3Human Dimension Division, HQ TRADOC, Fort Eustis, Fort Eustis, VA, United States, 4U.S. Army Aeromedical Research Laboratory, Fort Rucker, Fort Rucker, AL, United States, 5Department of Psychology, Auburn University, Auburn, AL, United States, 6Department of Psychology, Westfield State University, Westfield, MA, United States, 7Alabama Advanced Imaging Consortium, Auburn University and University of Alabama Birmingham, Birmingham, AL, United States
Synopsis
Conscious regulation of emotions is essential for sound functioning of an individual, while its disruption leads to several severe symptoms observed in psychiatric disorders like posttraumatic stress disorder (PTSD) and mild-traumatic brain injury (mTBI). While the brain regions activated in emotion regulation have been elucidated in prior works, an understanding of the underlying network has been elusive. Employing an emotion regulation task, we discovered the network of emotion regulation in healthy soldiers, and dysregulation in soldiers with comorbid PTSD/mTBI (N=59). Our work is significant given that we present, for the first time, the evidence for the network of emotion regulation/dysregulation.
Introduction
Emotions play an important role in our healthy functioning. Our ability to shape our emotion experience is known as emotional regulation [1], involving voluntary modification of emotions elicited in response to exogenous stimuli. Several functional MRI activation studies have consistently identified the middle frontal gyrus (MFG), anterior cingulate and insula to be involved in emotion regulation [1], but their limitation lies in the inability to explain the interrelationship between these regions, i.e. connectivity. The brain network of emotion regulation either in healthy adults or in psychiatric disorders like posttraumatic stress disorder (PTSD) and mild-traumatic brain injury (mTBI) has been elusive. Emotion dysregulation has been regarded as a primary cause for several symptoms observed in PTSD and mTBI [2]. Using fMRI data collected during an emotion regulation task, we obtained in a data-driven fashion, the network of emotion regulation in healthy soldiers and dysregulation in soldiers with comorbid PTSD and chronic mTBI (or post-concussion syndrome [PCS]).Methods
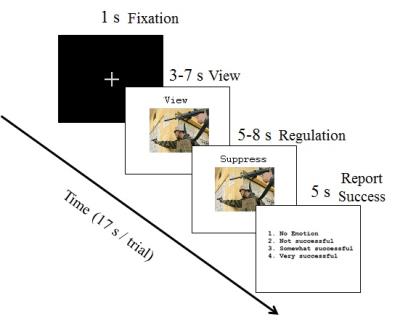
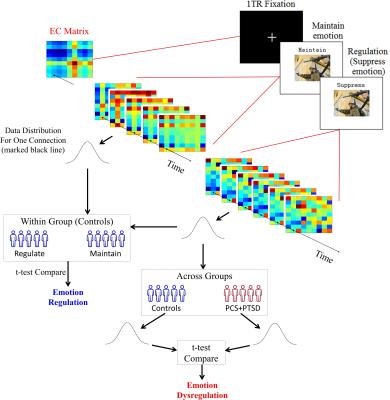
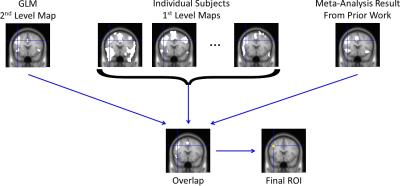
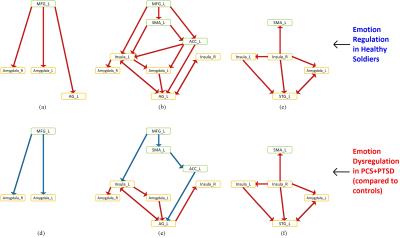
Fifty-nine male U.S. Army soldiers were recruited (comorbid PTSD and PCS [PCS+PTSD]=36, combat controls=37, matched in age, race and education). FMRI data was acquired in a Siemens Magnetom Verio 3T scanner (EPI sequence, TR/TE=600/30ms, flip-angle=55o, multiband-factor=2, voxel-size=3.5×3.5×5mm3). The emotion regulation task (Fig.1) was similar to Urry et.al. [3]. Participants were presented images which either elicited a neutral or a negative emotional response. They were instructed to either “maintain” their emotional response, or “suppress” it (reduce intensity of negative feelings, requiring emotion regulation). There were 4 task blocks, with 24-trials in each block (4-trials, 6-repetitions). The comparison between (i) negative-image, suppress-emotion and (ii) negative-image, maintain-emotion would inform us on the neural mechanisms underlying emotion regulation. Standard pre-processing was performed in SPM [4] (realignment, smoothing [8mm-kernel], normalization to MNI-space, quality control). We first performed activation analysis to identify significantly activated regions during emotion regulation (see Fig.2 for region selection procedure). Hemodynamic deconvolution was performed [5] on mean time-series extracted from identified regions, to minimize the non-neural intra-subject HRF variability [6]. Given that voluntary emotion regulation is a top-down process [1], we employed effective connectivity (EC) modeling using Granger causality (GC) [7] to assess directional causal relationships between identified regions, similar to recent works [8, 9, 10]. Subject-wise EC between all regions were obtained, using which the networks of emotion regulation in healthy soldiers (‘suppress’ vs ‘maintain’ negative emotion) and its impairment in PCS+PTSD (control vs PCS+PTSD for ‘suppress’ negative emotion condition) were obtained (p<0.001, Bonferroni corrected) (Fig.3). We provide novel evidence for the brain networks of both voluntary emotion regulation, and its dysregulation in a clinical population.Results and Discussion
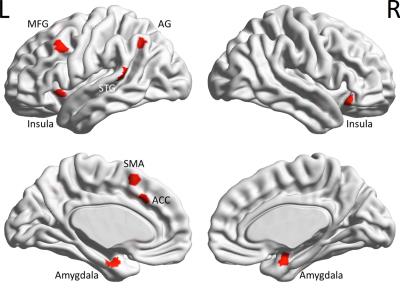
In this work, we investigated brain networks of emotion regulation in healthy soldiers, and dysregulation in comorbid PCS and PTSD. We identified regions activated during the emotion regulation task, and defined ROIs around the centroid of each of the nine identified clusters (Fig.4). Performing EC analysis, we identified the regulation network as having a top-down architecture with the MFG driving the rest of the network (insula, medial prefrontal regions, amygdala and lateral parietal regions) (Figs 5a,5b,5c). During dysregulation this network was imbalanced with reduction in prefrontal connectivity and elevation of subcortical and lateral parietal connectivity (Figs 5d,5e,5f). Our network of emotion regulation fits well with findings from prior studies [1, 11, 12], which have identified the pivotal role of MFG in the initiation of emotion regulation. Although amygdala facilitates emotion generation, and medial prefrontal regions facilitate subconscious emotion regulation like fear conditioning [1], the MFG initiates conscious cognitive emotion regulation [1]. MFG is implicated in executive functions like cognitive control [13], which are necessary for regulating emotions. Soldiers with PTSD have been shown to have impaired cognitive functions associated with the MFG [14], as well as impaired emotional processing [15]. All our directional connections are traceable to the MFG, implying that MFG could be the source of emotion regulation [1]. As for dysregulation, the MFG emerged as the key source of dysfunction in soldiers with PCS+PTSD. All connections emerging from MFG exhibited reduced connectivity, whose “ripple-effect” culminated in disinhibition of amygdala, which might translate into elevated retrieval of undesirable traumatic memories, causing symptoms like flashbacks, trauma re-experiencing and hyperarousal. This explanation fits well with behavioral manifestations of these conditions [2]. In summary, we identified the MFG to be pivotal during emotion regulation in healthy soldiers and dysregulation in soldiers with PCS+PTSD. Our results are significant given that these regions are implicated in prior activation studies [16, 17, 11], but a precise understanding of the underlying network structure and their causal relationships had not emerged so far.Acknowledgements
The authors acknowledge financial support for this work from the U.S. Army Medical Research and Materiel Command (MRMC) (Grant # 00007218). The views, opinions, and/or findings contained in this article are those of the authors and should not be interpreted as representing the official views or policies, either expressed or implied, of the U.S. Army or the Department of Defense (DoD). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors thank the personnel at the TBI clinic and behavioral health clinic, Fort Benning, GA, USA and the US Army Aeromedical Research Laboratory, Fort Rucker, AL, USA, and most of all, the soldiers who participated in the study. The authors thank Julie Rodiek and Wayne Duggan for facilitating data acquisition.References
[1] J. J. Gross, Handbook of Emotion Regulation, Ney York: The Guilford Press, 2014.
[2] M. E. Costanzo, Y. Y. Chou, S. Leaman, D. L. Pham, D. Keyser, D. E. Nathan, M. Coughlin, P. Rapp and M. J. Roy, "Connecting combat-related mild traumatic brain injury with posttraumatic stress disorder symptoms through brain imaging," Neuroscience Letters, vol. 577, pp. 11-15, 2014.
[3] H. L. Urry, C. M. van Reekum, T. Johnstone, N. H. Kalin, M. E. Thurow, H. S. Schaefer, C. A. Jackson, C. J. Frye, L. L. Greischar, A. L. Alexander and R. J. Davidson, "Amygdala and Ventromedial Prefrontal Cortex Are Inversely Coupled during Regulation of Negative Affect and Predict the Diurnal Pattern of Cortisol Secretion among Older Adults," The Journal of Neuroscience, vol. 26, no. 16, pp. 4415-4425, 2006.
[4] K. J. Friston, J. Ashburner, S. J. Kiebel, T. E. Nichols and W. D. Penny, Statistical Parametric Mapping: The Analysis of Functional Brain Images, Academic Press, 2007.
[5] M. Havlicek, K. J. Friston, J. Jan, M. Brazdil and V. D. Calhoun, "Dynamic modeling of neuronal responses in fMRI using cubature Kalman filtering," Neuroimage, vol. 56, no. 4, pp. 2109-28, 2011.
[6] D. A. Handwerker, J. M. Ollinger and M. D'Esposito, "Variation of BOLD hemodynamic responses across subjects and brain regions and their effects on statistical analyses," Neuroimage, vol. 21, no. 4, pp. 1639-51, 2004.
[7] G. Deshpande, K. Sathian and X. Hu, "Assessing and compensating for zero-lag correlation effects in time-lagged Granger causality analysis of FMRI," IEEE Transactions on Biomedical Engineering, vol. 57, no. 6, pp. 1446-56, 2010.
[8] M. D. Wheelock, K. R. Sreenivasan, K. H. Wood, L. W. Ver Hoef, G. Deshpande and D. C. Knight, "Threat-related learning relies on distinct dorsal prefrontal cortex network connectivity," Neuroimage, vol. 102, no. 2, pp. 904-12, 2014.
[9] G. Deshpande, L. E. Libero, K. R. Sreenivasan, H. D. Deshpande and R. K. Kana, "Identification of neural connectivity signatures of autism using machine learning," Frontiers in Human Neuroscience, vol. 17, p. 7:670, 2013.
[10] N. L. Hutcheson, K. R. Sreenivasan, G. Deshpande, M. A. Reid, J. Hadley, D. M. White, L. Ver Hoef and A. C. Lahti, "Effective connectivity during episodic memory retrieval in schizophrenia participants before and after antipsychotic medication," Human Brain Mapping, vol. 36, no. 4, pp. 1442-57, 2015.
[11] N. Kohn, S. B. Eickhoff, M. Scheller, A. R. Laird, P. T. Fox and U. Habel, "Neural network of cognitive emotion regulation--an ALE meta-analysis and MACM analysis," Neuroimage, vol. 87, pp. 345-55, 2014.
[12] A. Zilverstand, M. Parvaz and R. Goldstein, "Neuroimaging cognitive reappraisal in clinical populations to define neural targets for enhancing emotion regulation. A systematic review," Neuroimage, p. (in press), 2016 June 7.
[13] K. Emmert, R. Kopel, J. Sulzer, A. B. Brühl, B. D. Berman and et al., "Meta-analysis of real-time fMRI neurofeedback studies using individual participant data: How is brain regulation mediated?," Neuroimage, vol. 124, no. Pt A, pp. 806-12, 2016.
[14] M. Dretsch, K. Thiel, J. Athy, C. Irvin, B. Fjordbak and A. Salvatore, "Mood symptoms contribute to working memory decrement in active-duty soldiers being treated for posttraumatic stress disorder," Brain & Behavior, vol. 2, no. 4, pp. 357-364, 2012.
[15] M. Dretsch, K. Thiel, J. Athy, S. Born and K. Prue-Owens, "Posttraumatic Stress Disorder in the U.S. Warfighter: Sensitivity to Punishment and Antidepressant Use Contribute to Decision-Making Performance," Traumatology, vol. 19, pp. 118-125, 2012.
[16] A. N. Simmons and S. C. Matthews, "Neural circuitry of PTSD with or without mild traumatic brain injury: a meta-analysis," Neuropharmacology, vol. 62, no. 2, pp. 598-606, 2012.
[17] J. Hayes, M. Vanelzakker and L. Shin, "Emotion and cognition interactions in PTSD: a review of neurocognitive and neuroimaging studies," Frontiers in Integrated Neuroscience, vol. 6, p. 89, 2012.
Figures