Michael Albert Thomas1, Rajakumar Nagarajan2, Eric S Daar3, Santosh K Yadav4, Charles H Hinkin5, Manoj K Sarma6, Zohaib Iqbal1, Sathya Arumugam1, Mario Guerrero 3, Mohammad Haris4, and Ebrahim Haroon7
1Radiological Sciences, UCLA Geffen School of Medicne, los angeles, CA, United States, 2Radiological Science, UCLA Geffen School of Medicine, los angeles, CA, United States, 3Medicine, Harbor-UCLA Medical Center, Torrance, CA, United States, 4Research Branch, Sidra Medical and Research center, Doha, Qatar, 5Psychiatry, UCLA Geffen School of Medicine, Los Angeles, CA, United States, 6Radiological Sciences, UCLA Geffen School of Medicine, Los Angeles, CA, 7Psychiatry, Emory University, Atlanta, GA, United States
Synopsis
Regional brain volumes and cortical thickness using
3D T1-weighted MP-RAGE and neurometabolites quantitated using 5D
EP-JRESI MRSI were obtained from a group of HIV+ (n=16) and HIV-subjects (n=15). Compared to HIV- subjects, following findings were
observed in HIV+: i) decreases in the volume of right thalamus, mid anterior
corpus callosal region and cortex (right, left, combined), and ii) decreases in
cortical thickness of superior parietal and inferior temporal regions. The
cortical thickness and volumetric changes were predicted by (increased choline,
decreased NAA and Glx). Right basal ganglia glutamate/glutamine ratios and HIV+
status together significantly predicted psychomotor slowing during
neurocognitive testing.
Purpose
More than 1.1 million people with HIV infection
are living in the United States1. Persistent and sometimes progressive
neurological dysfunction may stem from chronic, non-resolving host responses,
including persisting immune activation despite adequate viral suppression.
There remains a need to develop and refine new-generation technologies that can
help track the progress in underlying pathophysiology. MR imaging techniques
including MRI and MR spectroscopy facilitate non-invasive anatomical and
biochemical characterization2-4. A major goal of this work was to determine the
relationship between multiple neuroimaging parameters (3D T1-weighted
MP-RAGE and 5D EP-JRESI) and other markers of disease such as HIV disease
severity, immune activation and neurocognitive function. HIV disease severity
parameters have been described in a companion abstract and this will primarily
focus on the association between MRS and structural MR findings. Methods
Sixteen HIV+ subjects
(43.5±9.4 years) and 15 age-matched healthy subjects (46.3±9.7 years) were
recruited between January 2015 until July 2016. All subjects signed the consent
form approved by the institutional review board before participating in the
study. A 3T Skyra MRI scanner and a 16-channel head ‘receive’ coil were used. The
neuroimaging sequences included the following: 1) 3D MP-RAGE: TR/TE = 2300 ms/2.98 ms; average =1, FOV =256, T=900ms, flip angle =90,
matrix = 256x256, slices =176, bandwidth (BW) =240Hz. 2) accelerated
5D echo-planar-J-resolved spectroscopic Imaging (EP-JRESI)5: TE/TR = 30/1200 ms, FOV = 24x24x12 cm3,
1.5x1.5x1.5 cm3 resolution, spectral BW (F2/F1)=
1190/1000 Hz, 64 t1 increments, 8x NUS for a scan time ~20 min. 3)
Neurocognitive testing were performed by a previously validated mini neuropsychological
test battery including attention, psychomotor speed, memory and construction. The
FreeSurfer program (version 5.3) was used to
quantify cortical and sub-cortical volume changes6-7. The 5D EP-JRESI data was reconstructed as explained
recently5.Results
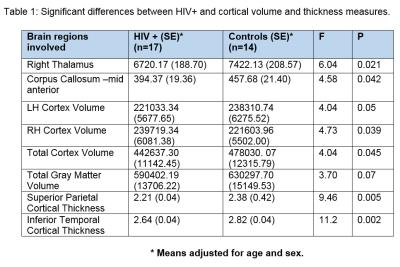
Comparison of regional volume and cortical
thickness changes in HIV versus Controls:
HIV+ individuals were compared to age and sex
matched healthy controls and the results of the analysis is shown in Table 1.
HIV+ individuals demonstrated decreases in the volume of right thalamus, mid
anterior corpus callosal region and cortex (right, left, combined). HIV+
individuals also showed decreases in cortical thickness parameters involving
superior parietal and inferior temporal regions. Association between metabolites
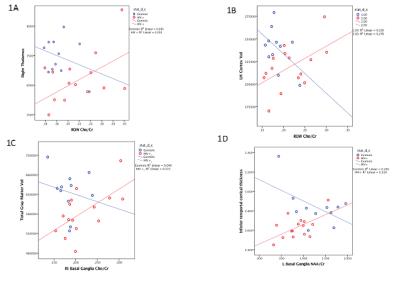
and cortical volume and thickness changes: Higher Ch/Cr ratios in the
right occipital white matter regions predicted larger right thalamic volumes
(Figure 1A), cortical volumes (right, left and bi hemispheric, only left shown
Figure 1B); while right basal ganglia Ch/Cr predicted total gray matter volume
(Figure 1C) and left basal ganglia NAA/Cr predicted cortical thickness of
inferior temporal cortex (Figure 1D). All above associations were seen only in
the HIV subjects and not in healthy controls. Association between psychomotor
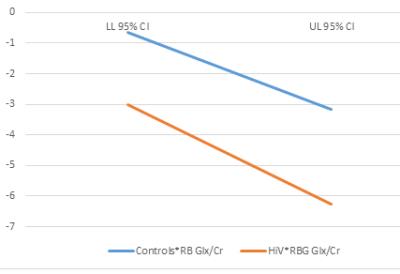
speed and right basal ganglia glutamate: A multiple linear regression analysis showed that right basal
ganglia glutamate (RB Glx/Cr) and HIV+ status were the only significant
predictors of psychomotor slowing measured using a mini neurocognitive test
battery. In order to measure the combined effect of RB Glx/Cr and HIV+ status a
mixed model analysis was performed to test the combined effects HIV+ status*RB
Glx/Cr. The HIV+Status*RB Glx/Cr interaction term was significantly associated
with decreases in psychomotor speed [F(1,4)=5.714, p=0.009]. Figure 2 shows the
parameter estimates with 95% of combined effect of HIV+*RB Glx/Cr on
psychomotor slowing. Discussion
HIV+ patients demonstrated significant volumetric
decreases in subcortical (thalamic), white matter (corpus callosum) and
cortical (right, left and combined) and decreases in gray matter thickness in
superior parietal and inferior temporal cortices. The association of these
volumetric and cortical thickness changes with concurrent increases in Ch/Cr
and decreases in NAA/Cr resonances indicate increased glial reactivity in the
presence of neuronal loss. The cortical thickness and volumetric changes were
predicted by metabolic changes in regions known to be connected to the
regions-of-interest. Behavioral changes such as loss of psychomotor speed seen
among many HIV afflicted individuals are related to a combined impact of HIV+
status and glutamate increases in the basal ganglia. Conclusion
This
pilot study demonstrates the association between the volumes of different brain
regions and cortical thinning derived from the 3D MP-RAGE analysis, brain
chemical changes in multiple locations using the 5D EP-JRESI data and neurocognitive
changes reflected through decreased psychomotor speed. These data provide key
support to the usefulness of multi-modality MR imaging techniques in assessing
brain changes in HIV+ individuals.Acknowledgements
NIH/NINDS grant (1R21NS086449-01A1). References
1) Spudich SS, Ances BM. Neurologic
complications of HIV infection. Top Antivir Med. 2012 Jun-Jul;20(2):41
2) Lentz MR, Kim WK, Lee V, et al. Changes in MRS neuronal
markers and T-cell phenotypes observed
during
early HIV infection. Neurology 2009; 72:1465-72
3) Sailasuta N, Ross W, Ananworanich J, Clemchai T, DeGruttola
V, Lerdlum S, Pothisri M, Busovaca E,
Ratto-Kim S, Jagodzinski L, Spudich S, Michael
N, Kim JH, Valcour V RV254/SEARCH 010
protocol
teams.Change in brain magnetic resonance spectroscopy after treatment during
acute HIV
infection. PLoS One2012;7:e49272
4) Young AC, Yiannoutsos CT, Hegde M, Lee E, Petersen J, Walter R, Price
RW, Meyerhoff DJ, Spudich S. Cerebral metabolite changes prior to and after
antiretroviral therapy in primary HIV infection. Neurology 2014,
83:1592-600.Epub 2014 Sep.26
5) Wilson, et al., Accelerated
Five-Dimensional Echo Planar J-Resolved Spectroscopic Imaging: Implementation
and Pilot Validation in Human Brain.Magn
Reson Med, 2016;75:42-51
6) Yadav SK, Gupta RK, Saraswat VA, Reduced
cortical thickness in patients with acute-on-chronic liver failure due to
non-alcoholic etiology. J Trans Med 2015;13:322
7) Yadav SK, Kathiresan N, Mohan S, et al.
Gender-based analysis of cortical thickness and structural connectivity in
Parkinson’s disease. See comment in PubMed Commons belowJ Neurol. 2016
Nov;263(11):2308-2318. Epub 2016 Aug 20