0497
Function versus occupancy in the human brain: PET/fMRI during infusion of D2 antagonist1Psychiatry, Massachusetts General Hospital and Athinoula A. Martinos Center for Biomedical Imaging, Boston, MA, United States, 2Harvard Medical School, Boston, MA, United States, 3Radiology, Massachusetts General Hospital and Athinoula A. Martinos Center for Biomedical Imaging, Boston, MA, United States, 4Radiology, Harvard Medical School, Boston, MA, United States, 5Psychiatry, Harvard Medical School, Boston, MA, United States
Synopsis
Simultaneous PET and fMRI were employed in healthy human subjects to investigate the dose-dependent relationship between drug occupancies of a D2-receptor antagonist and induced CBF responses measured by arterial spin labeling. Results indicate a super-linear relationship between CBF and occupancy, with a larger CBF response in putamen than in caudate at matched occupancies. These results inform dopaminergic neurophysiology, and the method may provide general utility for probing dopaminergic function in human subject cohorts.
Purpose
The dopamine-rich basal ganglia play prominent roles in
movement disorders like Parkinson’s disease, psychiatric disorders including
schizophrenia, behaviors related to natural rewards and drug abuse, and aspects
of pain and depression. Previously, estimates of basal dopamine levels in the
human brain have been derived by comparisons of binding potentials (BPs) of
dopaminergic radioligands before and after pharmacological depletion of nearly
all dopamine (1,2),
an approach that induces severe side effects over a time period of days. This
study sought to translate a preclinical method for potentially probing basal
dopamine occupancy based upon simultaneous PET and fMRI measurements during
infusion of a D2 antagonist (3).
In this method, fMRI reports a functional response induced by dopamine
displacement from D2 receptors during simultaneous measurement of PET
occupancy, and the main outcome variable is the fMRI signal magnitude per unit
occupancy.
Methods
Healthy adult volunteers (n=6) were recruited for a single-visit imaging study using simultaneous PET and fMRI in conjunction with i.v. infusion of prochlorperazine within clinical dose limits. The aims of this study were to investigate the dose-response to the drug, subject tolerance to side effects within the imaging environment, and fMRI and PET detection sensitivities versus dose. In each study, prochlorperazine was infused over 2 minutes at a time no less than 30 minutes after injection of [11C]raclopride, and PET imaging continued for 90 minutes in order to dynamically assess changes in drug occupancy at D2/D3 receptors. Concurrently, a multi-slice pseudo-continuous arterial spin labeling (pCASL) pulse sequence (TR/TE 4000/12, label duration 1600) recorded changes in cerebral blood flow (CBF) throughout the imaging session. PET and fMRI data were modeled, and occupancy and induced change in CBF were determined as modeled responses at 40 minutes post-drug.Results
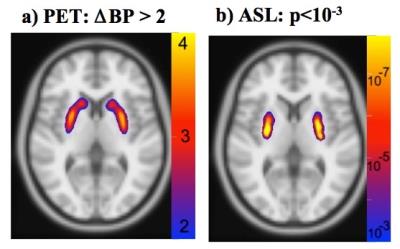
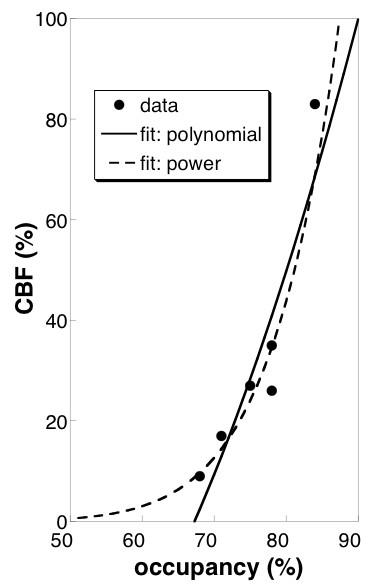
Doses of prochlorperazine ranged from 20-50 ug/kg (1.4 to 4.0 mg), compared to the clinical dose limit of 10 mg. Subjects tolerated the drug well; no subjects exhibited or reported akathisia. Drug occupancies at D2/D3 receptors in putamen ranged from about 70% at lower doses to about 85% at the highest dose. Dynamic PET indicated a slow temporal increase in drug occupancy, with an average sigmoidal time constant of about 25 minutes. pCASL detected robust increases in CBF in basal ganglia across sessions (Fig. 1b), whereas BOLD signal derived from the pCASL series did not show significant activation (not shown). Drug-induced CBF responses were larger in putamen than in caudate, consistent with preclinical results using this method (3) and with invasive measurements that report higher dopamine levels in putamen (4-6). The relationship between CBF and occupancy (Fig. 2) suggests that functional changes are superlinear versus occupancy, as observed preclinically, or that CBF changes respond above an occupancy threshold.Discussion
The ability to simultaneously measure changes in neuro-receptor occupancy and brain function by PET/fMRI offers a wide range of potential applications, but clinical translation is challenging. This study translates a preclinical method for probing dopaminergic function by 1) utilizing a clinical drug that is well tolerated, and 2) using long periods of pCASL to track slow changes in CBF that are not captured well by BOLD signal. In this study, changes in PET binding potentials were easily measurable, and fMRI proved to be the limiting factor in detecting the response to drug. Although the sequence echo time was suboptimal for BOLD fMRI, results support the notion that ASL detection power exceeds BOLD signal at very low frequencies, where BOLD responses are indistinguishable from drift (7). Further improvements and distribution of state-of-the-art ASL methodologies can be expected to fill an important role for these types of clinical PET/fMRI research studies.
A strong increase in fMRI response above 70% occupancy is consistent with the notion of a “therapeutic window” for D2-receptor antagonists as they are employed clinically. This strong non-linearity in function-occupancy results (Fig. 2) could confound attempts to use the ratio of fMRI and PET measurements as an index of dopamine level, especially at lower occupancies. A previous preclinical PET/fMRI study measured a function-occupancy curve that was more linear than results obtained in this study to date (3). Additional measurements will be required to more completely characterize the curve.
Conclusion
These data provide direct correlations between D2/D3 receptor occupancy by antagonist and induced functional responses in human brain. Larger CBF responses in putamen than caudate are consistent with literature reports of higher dopamine levels in putamen than caudate. More data will be required to fully characterize the function-occupancy relationship, with the goal of cross-sectional comparisons using a fixed antagonist dose at relatively high occupancy.Acknowledgements
This research was supported by grants from the National Institutes of Health (R21NS090169, 5T32MH016259-35, P41EB015896, S10RR026666, S10RR022976, S10RR019933, S10RR017208). The authors declare no conflict of interest.References
1. Martinez D, Greene K, Broft A, Kumar D, Liu F, Narendran R, Slifstein M, Van Heertum R, Kleber HD. Lower level of endogenous dopamine in patients with cocaine dependence: findings from PET imaging of D(2)/D(3) receptors following acute dopamine depletion. American Journal of Psychiatry 2009;166:1170–1177. doi: 10.1176/appi.ajp.2009.08121801.
2. Abi-Dargham A, Rodenhiser J, Printz D, et al. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc Natl Acad Sci USA 2000;97:8104–8109.
3. Sander C, Hooker J, Catana C, Normandin M, Alpert N, Knudsen G, Vanduffel W, Rosen B, Mandeville J. Neurovascular coupling to D2/D3 dopamine receptor occupancy using simultaneous PET/functional MRI. Proceedings of the National Academy of Sciences 2013.
4. Skirboll S, Wang J, Mefford I, Hsiao J, Bankiewicz KS. In vivo changes of catecholamines in hemiparkinsonian monkeys measured by microdialysis. Exp Neurol 1990;110:187–193.
5. Moghaddam B, Berridge CW, Goldman-Rakic PS, Bunney BS, Roth RH. In vivo assessment of basal and drug-induced dopamine release in cortical and subcortical regions of the anesthetized primate. SYNAPSE 1993;13:215–222. doi: 10.1002/syn.890130304.
6. Goldstein DS, Sullivan P, Holmes C, Kopin IJ, Basile MJ, Mash DC. Catechols in post-mortem brain of patients with Parkinson disease. Eur J Neurol 2011;18:703–710. doi: 10.1111/j.1468-1331.2010.03246.x.
7. Wang J, Aguirre G, Kimberg D, Roc A, Li L, Detre J. Arterial spin labeling perfusion fMRI with very low task frequency. Magn Reson Med 2003;49:796–802.