0493
StreST: Myocardial Creatine Kinase Rate in Both Rest and Stress.1OCMR, CV Med RDM, University of Oxford, Oxford, United Kingdom
Synopsis
A method for reduced time and stress myocardial creatine kinase measurements using 31P-MRS at 3T is presented. The new method is validated in healthy controls and demonstrated in 27 obese and heart-failure patients. In addition, the existing TRiST method is applied in 63 subjects for the first time on a Siemens platform.
Introduction
The rate constant of myocardial creatine kinase exchange ($$$k_\mathrm{f}^\mathrm{CK}$$$) is a sensitive measure of heart failure.1 Obesity is associated with the development of heart failure, but its association with disease progression and prognosis is complex.2 Measuring the energetics of the obese subject’s heart under stress may help to resolve these relationships.
A phosphorus MRS based $$$k_\mathrm{f}^\mathrm{CK}$$$ measurement method, triple repetition time saturation transfer (TRiST), has previously been developed for 3T.3 TRiST takes 40 min to make a measurement.4 Myocardial stress can be induced by intravenous dobutamine, but administering dobutamine for >20 minutes is unsafe. To measure a stress $$$k_\mathrm{f}^\mathrm{CK}$$$ at 3T, a shortened protocol is needed. This is possible using the “Two repetition time saturation transfer” (TwiST) method;4 but this requires the assumption of a fixed phosphocreatine (PCr) intrinsic T1 ($$$T_1^*$$$), which is not safe to assume in different subject groups e.g. obese or heart-failure.
We propose using the T1* measured in a rest TRiST acquisition to run a reduced time second measurement during dobutamine induced stress. The new, shorter, “StreST” protocol is validated in healthy volunteers, it is subsequently applied in 27 normal and heart-failure volunteers.
Theory
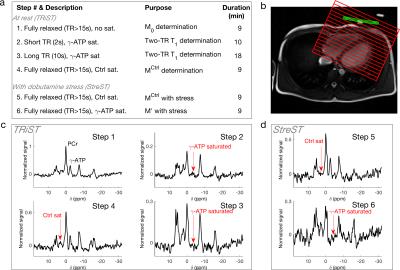
TRiST utilises steady-state saturation transfer of γ-ATP as it undergoes exchange with PCr. During steady-state saturation the rate constant of exchange is $$k_\mathrm{f}^\mathrm{CK}=\frac{1}{T_1'}\left(1-\frac{M_\mathrm{PCr}'}{M_\mathrm{PCr}^\mathrm{Ctrl}}\right),$$ where $$$T_1'$$$ is the saturation affected $$$T_1^\mathrm{PCr}$$$, $$$M_\mathrm{PCr}'$$$ is the saturation affected magnetisation of PCr, and $$$M_\mathrm{PCr}^\mathrm{Ctrl}$$$ is the magnetisation with mirrored control saturation. In TRIST these are determined in three steps (Fig. 1a). $$$T_1^*$$$ may be calculated thus:$$T_1^*=T_1'\frac{M_\mathrm{PCr}^\mathrm{Ctrl}}{M_\mathrm{PCr}'}.$$By substituting Eq. [2], Eq. [1] may be recast in terms of $$$T_1^*$$$ rather than $$$T_1'$$$:$$k_\mathrm{f}^\mathrm{CK}(\mathrm{Stress})=\frac{1}{T_1^*}\left(\frac{M_\mathrm{PCr}^\mathrm{Ctrl}(\mathrm{Stress})}{M_\mathrm{PCr}'(\mathrm{Stress})}-1\right).$$If $$$M_\mathrm{PCr}'(\mathrm{Stress})$$$, $$$M_\mathrm{PCr}^\mathrm{Ctrl}(\mathrm{Stress})$$$ are determined directly, $$$k_\mathrm{f}^\mathrm{CK}(\mathrm{Stress})$$$ can be measured in two steps in subsequent scans on the same subject.Methods
Experiments used a 3T Trio (Siemens, Germany). The body coil was used for 1H localisation. A 10-cm loop, phosphorus tuned, transmit-receive surface coil (Pulse Teq, UK) was used for spectroscopy.
Validation
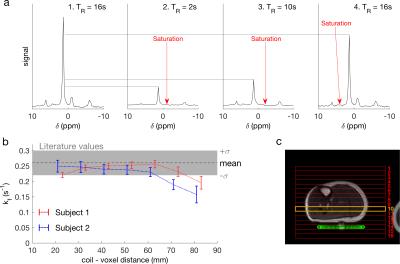
The single-measurement TRiST implementation was tested in calf skeletal muscle, in which $$$k_\mathrm{f}^\mathrm{CK}$$$ has been widely measured5, of two subjects (Fig. 2).
The consecutive reduced-time $$$k_\mathrm{f}^\mathrm{CK}$$$ measurement was tested in six healthy volunteers. After a TRiST measurement (steps 1-4, Fig. 1) a second measurement was made, without any change in the subject’s condition (i.e. both at rest), using the StreST protocol (steps 5&6, Fig. 1).
Experiment
The full protocol (steps 1-6), including dobutamine stress during steps 5&6, was run in 27 age-matched subjects (4 normal weight healthy, 14 obese healthy, 3 normal weight heart failure, 6 obese heart failure patients). An additional 12 normal-weight subjects and 16 normal-weight heart-failure patients underwent the TRiST protocol (steps 1-4).
Acquisition
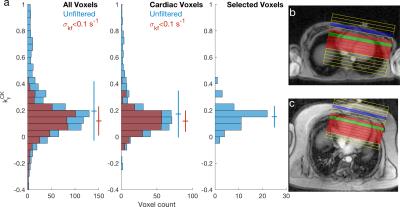
TRiST/StreST used 1D-CSI with FOV = 160 mm, 16 matrix, 3 kHz bandwidth, 512 samples; CSI grid positioned perpendicular to a transverse heart localiser and parallel to the chest wall. Excitation used a 7.5-ms AHP pulse (210 V) and saturation used DANTE (voltage maximised for SAR, approx. 30V). Myocardial spectra were selected using a transverse 1H localiser. The data from the most apical voxel, corresponding to a coil-to-voxel distance of <60 mm, was analysed separately.
Results
In the skeletal muscle, $$$k_\mathrm{f}^\mathrm{CK}$$$ measured in voxels 10-60 mm away from the coil were consistent with values reported from the literature (Fig. 2).5
Across all myocardial voxels, in all subjects (total: 63), measured using TRiST, the mean (±SD) $$$k_\mathrm{f}^\mathrm{CK}$$$ is 0.17±0.18 s-1, if voxels are filtered for an estimated uncertainty in $$$k_\mathrm{f}^\mathrm{CK}$$$ of <0.1 s-1 the mean $$$k_\mathrm{f}^\mathrm{CK}$$$ is 0.12±0.08 s-1. Using only the most anterior voxel in myocardium (Fig. 3) the mean $$$k_\mathrm{f}^\mathrm{CK}$$$ is 0.14±0.08 s-1.
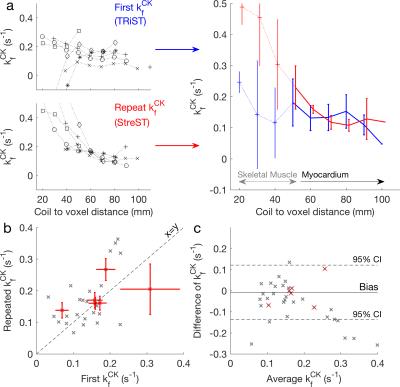
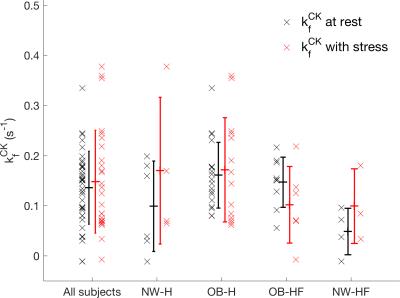
The correlation between the first and repeated measurement in validation (Fig. 4) was 0.51 for myocardial voxels and 0.65 for the selected voxels (Fig. 4c). There was 0.01 s-1 bias between the methods, but the coefficient of reproducibility was 0.12 s-1 (Fig. 4d). The results of the rest-stress experiments are shown in Figure 5.
Discussion
The method was validated successfully in skeletal muscle, matching the literature values closely in a large region of tissue. However, in myocardium the measured $$$k_\mathrm{f}^\mathrm{CK}$$$ was significantly lower than that reported by Johns Hopkins.3,4,6,7 Care was taken to match the original method, though hardware differences (e.g. the RF-coil) remain.
The rest-stress experiment was demonstrated, resulting in an unbiased, though variable, second measurement under stress. StreST has so far been used in 27 external subject scans, including obese subjects and heart-failure patients.
Conclusion
We have demonstrated the StreST method for stress creatine-kinase rate measurements in healthy and heart-failure subjects. In addition, we present the first known implementation of TRiST outside the originating site.Acknowledgements
We thank Michael Schar for the help he provided in implementing TRiST.
Funding sources: A Sir Henry DaleFellowship from the Wellcome Trust and the Royal Society, 098436/Z/12/Z; BHF Clinical Research Training Fellowship, FS/14/54/30946.
References
1. Weiss RG, Gerstenblith G, Bottomley PA. ATP flux through creatine kinase in the normal, stressed, and failing human heart. Proc Natl Acad Sci U S A 2005;102(3):808-813.
2. Rayner JJ, Neubauer S, Rider OJ. The paradox of obesity cardiomyopathy and the potential for weight loss as a therapy. Obes Rev 2015;16(8):679-690.
3. Schar M, El-Sharkawy AMM, Weiss RG, Bottomley PA. Triple Repetition Time Saturation Transfer (TRiST) (31)P Spectroscopy for Measuring Human Creatine Kinase Reaction Kinetics. Magn Reson Med 2010;63(6):1493-1501.
4. Schar M, Gabr RE, El-Sharkawy AM, Steinberg A, Bottomley PA, Weiss RG. Two repetition time saturation transfer (TwiST) with spill-over correction to measure creatine kinase reaction rates in human hearts. J Cardiov Magn Reson 2015;17.
5. Valkovic L, Chmelik M, Kukurova IJ, Krssak M, Gruber S, Frollo I, Trattnig S, Bogner W. Time-resolved phosphorous magnetization transfer of the human calf muscle at 3 T and 7 T: A feasibility study. Eur J Radiol 2013;82(5):745-751.
6. Smith CS, Bottomley PA, Schulman SP, Gerstenblith G, Weiss RG. Altered creatine kinase adenosine triphosphate kinetics in failing hypertrophied human myocardium. Circulation 2006;114(11):1151-1158.
7. Bottomley PA, Wu KC, Gerstenblith G, Schulman SP, Steinberg A, Weiss RG. Reduced Myocardial Creatine Kinase Flux in Human Myocardial Infarction An In Vivo Phosphorus Magnetic Resonance Spectroscopy Study. Circulation 2009;119(14):1918-1924.
Figures