0491
Detection of Carbonic Anhydrase Activity in Human Brain in Vivo1National Institutes of Health, Bethesda, MD, United States
Synopsis
This study demonstrates the feasibility of detecting carbonic anhydrase activity in the human brain. A very large magnetization transfer effect on the bicarbonate signal was measured in healthy human subjects using 13C MRS upon saturation of carbon dioxide. Despite the large variations in blood glucose response to oral administration of 13C-labeled glucose the magnetization transfer effect was measured with high precision.
Purpose
In brain, carbonic anhydrase (CA) plays a vital role in long-term synaptic transformation and attentional gating of memory storage. Carbonic anhydrase dysfunction is known to be associated with aging, mental retardation and Alzheimer's disease1. Previously, carbonic anhydrase activity was measured in vivo in the rat brain using 13C magnetization transfer MRS2. Here we demonstrate that carbonic anhydrase activity can be measured in the human brain in vivo using 13C magnetization transfer.Methods
Hardware: All studies were performed on a Siemens 7T scanner using a home-built coil assembly. The proton coil was a shielded quadrature half-volume coil (two overlapping octagon loops, nominal size=12.7x12.7 cm2). The 13C coil was a 7-cm surface coil.
Glucose administration: Healthy volunteers were asked to fast overnight before the scan. Solution of 20% w/w 99% enriched [U-13C6]glucose was orally administrated to subject (n=3; 0.75 g [U-13C6]glucose per kg of body weight)3 right before scan started. One antecubital vein was cannulated for withdrawing blood every ten minutes to monitor blood glucose level.
13C MRS: A 5x5x5 cm3 cubic voxel in the occipital lobe was shimmed using Siemens 3D shimming method. All first- and second-order shims and five third order shims were adjusted. The typical half-height water linewidth from the cube was 14~16Hz. The pulse sequence is similar to that used in Ref. 2. TR=30 s, SW=8 kHz, data point=2048, NA=16 or 24, 13C excitation hard pulse duration=0.25 ms. No proton decoupling was applied. The RF preparation during TR consisted of proton hard pulses for NOE and 13C saturation at the carbon dioxide resonance of 125.0 ppm with γB1=50 Hz. The control spectra and carbon dioxide saturation spectra were interleaved. Each set of interleaved spectra (8 or 12 odd-numbered spectra with saturation of carbon dioxide and 8 or 12 even-numbered control spectra) took 8 or 12 min. The 13C excitation pulse was placed at bicarbonate frequency (160.7 ppm). The average input power from all RF pulses was less than 3W.
Results
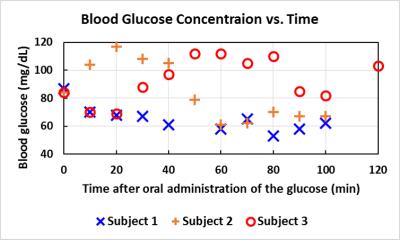
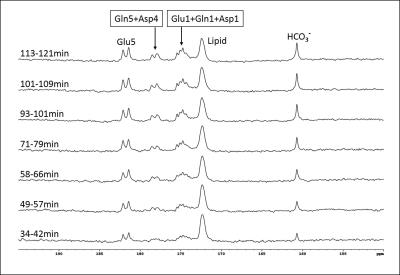
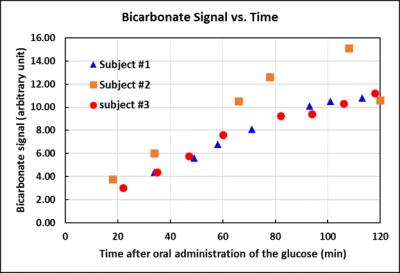
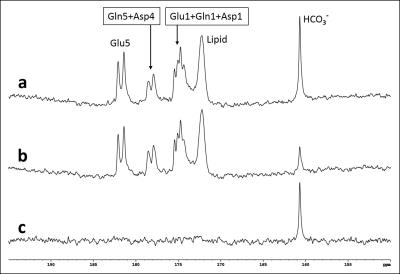
Blood glucose level responding to oral administration of glucose varied among the subjects as shown in Fig 1. Fig 2 shows time-course spectra from a typical subject after oral administration of [U-13C6]glucose without saturation of carbon dioxide. After oral administration of exogenous [U-13C6]glucose, 13C label incorporation into glutamate C5, glutamine C5, aspartate C4 as well as bicarbonate and the C1 resonances of glutamate, glutamine and aspartate were observed. Fig 3 shows the time course of bicarbonate signal intensity from three subjects. Fig. 4 shows the magnetization transfer effect catalyzed by carbonic anhydrase in the human brain between 110-120 min after oral administration. The ratio of the bicarbonate signal intensity with carbon dioxide saturation to that without carbon dioxide saturation was found to be 0.27 ± 0.035 (n=3). All in vivo spectra were processed with zero fill=16 k and LB=8 Hz with no baseline corrections.Discussion
In CNS, carbonic anhydrase is primarily expressed in glial and choroid cells with no significant carbonic anhydrase activities in neurons. It has been proposed recently that under conditions of high neuronal activity, glial processing of carbon dioxide and transfer of energy is coupled with its high-affinity glutamate uptake and other transport processes at the glial and neuronal cell membranes4. The work shown here demonstrates that it is practically feasible to measure carbonic anhydrase activity in vivo in the human brain, making it possible to characterize this important enzyme in many brain disorders. As shown by Figs 2 and 3, the kinetics of 13C label incorporation into bicarbonate is remarkably consistent among the three subjects despite the large differences in their blood glucose time course following oral glucose administration. In particular, there is little variation in the bicarbonate saturation transfer intensity ratio. These results bode well for 13C labeling studies using oral instead of intravenous glucose administration. Our results show that saturation of carbon dioxide caused bicarbonate signal to decrease by ~73% in the brain of adult healthy subjects (Fig 4). This very large magnetization transfer effect makes measuring carbonic anhydrase reaction particularly amenable to clinical MRS research. To our best knowledge, this is the first report of detection of carbonic anhydrase catalysis in human brain.Acknowledgements
This work was supported by the Intramural Research Program of the National Institute of Mental Health, National Institutes of Health.
References
1. Sun MK, et al, Carbonic anhydrase gating of attention: memory therapy and enhancement. Trends Pharmacol Sci. 2002 Feb;23(2):83-9.
2. Xu S, et al, Studying enzymes by in vivo 13C magnetic resonance spectroscopy. Prog Nucl Magn Reson Spectrosc. 2009 Oct 1;55(3):266-283.
3. Mason GF, et al. A comparison of 13C NMR measurements of the rates of glutamine synthesis and the tricarboxylic acid cycle during oral and intravenous administration of [1-13C]glucose. Brain Research Protocols 2003;10:181.
4. Deitmer JW. A role for CO2 and bicarbonate transporters in metabolic exchanges in the brain. J Neurochem 2002;80:721–726.
Figures