0489
Multi-echo chemical shift encoding (MECSE) for sensitive fluorine-19 MRI of complex spectra1Radiology, Lausanne University Hospital (CHUV), Lausanne, Switzerland, 2Angiology, Lausanne University Hospital (CHUV), Lausanne, Switzerland, 3Advanced Clinical Imaging Technology, Siemens Healthcare AG, Lausanne, Switzerland, 4Radiology, University of Wisconsin-Madison, Madison, WI, United States, 5Medical Physics, University of Wisconsin-Madison, Madison, WI, United States
Synopsis
Now that several clinical trials are underway, sensitive fluorine-19 MRI of biocompatible perfluorocarbons with complex MR spectra, such as perfluorooctyl bromide (PFOB), is seeing increased interest. We here therefore propose a novel multi-echo chemical shift encoding (MECSE) technique that accounts for all resonances in k-space and reconstructs a high-sensitivity image free of chemical-shift-displacement artifacts. MECSE was developed and characterized in a phantom study, compared to established techniques for imaging compounds with complex spectra, and preliminarily demonstrated in vivo.
Introduction
Fluorine-19 (19F) MR imaging of perfluorocarbons emulsions (PFCs) is increasingly used for cell tracking and inflammation imaging in preclinical research1,2, while several clinical trials have recently also been initiated3. Unfortunately, the PFCs with the most promising biocompatibility profile such as perfluorooctyl bromide (PFOB)4 have complex MR spectra with multiple resonances. These resonances are often spread over a broad range (up to 10kHz) and create chemical shift displacement (CSD) artifacts that “dilute” the signal over multiple voxels. This decreases the already low signal-to-noise ratio (SNR) and confounds image interpretation.
Instead of attempting to suppress the additional resonances or to selectively excite some of them, we here propose a multi-echo chemical shift-encoded (MECSE) approach that combines a multi-echo acquisition with prior-knowledge-constrained image reconstruction. MECSE can in principle be used with any pulse sequence in which the echo time TE can be varied in small increments (on the order of 0.1ms) in a series of acquisitions.
The k-space signal sTE obtained with a MECSE 2D Cartesian pulse sequence can be modeled as:
$$s_{TE}(k_x,k_y(t),t)=\iint_{x,y}\rho (x,y) \big[\sum_{m=1}^{M}\alpha_me^{i2\pi(TE+t)f_m}\cdot C_m(TE+t)\big]e^{i2\pi xk_x}e^{i2\pi yk_y(t)}\,dx\,dy, \qquad (1)$$
where x and y are the phase-encoding and readout directions respectively, t is the time relative to the center of the current readout, ρ is the overall signal amplitude composed of M resonances with relative amplitudes αm and chemical shifts fm, and Cm describes additional known pulse-sequence-specific signal modulations (e.g. T1/T2/T2* relaxation behavior of each resonance, or banding effects in balanced steady-state free precession (bSSFP)).
To avoid CSD artifacts due to the large chemical shifts fm, the reconstruction can be formulated as a direct least-squares fit in k-space. Assuming prior knowledge about the spectral and relaxation properties of the compound of interest, the parameter map estimate ρ(x,y) that best fits the acquired k-space data sTE(kx,ky(t),t) is reconstructed5. Because the phase-encoding direction is not affected by CSD artifacts, the reconstruction can be solved separately for each location x.
The goal of this study was thus to implement the proposed MECSE approach for the spoiled gradient echo (GRE) and bSSFP pulse sequences, and to characterize its sensitivity relative to other recently reported sensitive 19F MRI techniques.
Methods
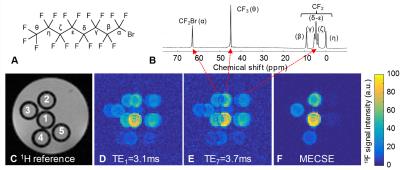
All experiments were performed at 3T (Magnetom Prisma, Siemens Healthcare) with a 19F/1H volume RF coil (Rapid Biomedical). A phantom was constructed from 5 syringes filled with agar and a range of PFOB concentrations, which were placed in a tube with agar.
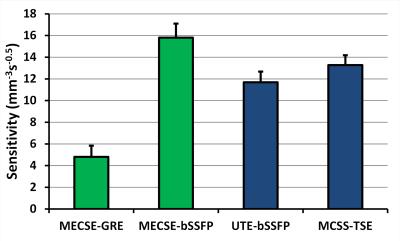
After 1H shimming, four prototype 19F pulse sequences were acquired: 1) 2D MECSE-GRE (TR=8.1ms, 7 TEs from 3.28 to 3.88ms with a 0.1ms increment, α=8°, voxel size V=0.5x0.5x2mm3, 64 averages, scan time Tacq=15min29s), 2) 2D MECSE-bSSFP (TR=6.7ms, 7 TEs from 3.1 to 3.7ms with a 0.1ms increment, α=60°, V=0.5x0.5x2mm3, 64 averages, Tacq=12min15s), 3) 3D bSSFP ultrashort echo time6 (bSSFP-UTE; TR=2.6ms, TE=90µs, α=30°, V=1x1x1mm3, 125952 lines, Tacq=4min30s), and 4) 3D multiple-chemical-shift-selective turbo spin echo7 (MCSS-TSE; TR=1700ms, TE=13.7ms, V=0.5x0.5x2mm3, Tacq=5min26s). Seven PFOB resonances were modeled for both MECSE techniques, and Cm in Equation 1 was established through Bloch equation simulations. The normalized sensitivity S of all pulse sequences was defined as:
$$S=SNR/(V\sqrt{T_{acq}}). \qquad (2)$$
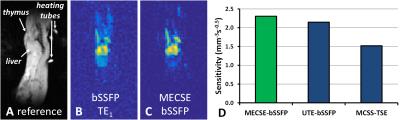
To demonstrate the equivalent sensitivity of MECSE in vivo, MECSE-bSSFP, bSSFP-UTE, and MCSS-TSE were repeated in two mice that were injected intraperitoneally with 300µl of PFOB 24h before imaging.
Results
MECSE was successfully implemented for both GRE and bSSFP imaging (Figure 1), and MECSE-bSSFP demonstrated higher sensitivity than the bSSFP-UTE and MCSS-TSE pulse sequences (Figure 2). The mouse studies furthermore confirmed these sensitivity differences (Figure 3). In some slices in both the phantoms and the mice, very small residual CSD artifacts could be detected, which were attributed to a combination of an imperfect RF pulse profile and residual magnetic field inhomogeneities.Discussion and Conclusions
The observed higher sensitivity of MECSE-bSSFP at 3T might be explained by several factors. While very fast, bSSFP-UTE uses less-sensitive 3D radial half-echo readouts of only the CF2 groups. Similarly, MCSS-TSE most likely performs sub-optimally at 3T due to the challenge in balancing a short TE with long RF pulses that separately excite the close γ-δε-ζ resonances (Figure 1B)7,8. In contrast, the MECSE approach was applied to fast Cartesian imaging techniques without any adverse constraints, although the bSSFP version might be faster and thus more sensitive at lower spatial resolutions. The extension of MECSE to 3D imaging is furthermore trivial, while its accuracy may be improved by including magnetic field inhomogeneities in the model.
In conclusion, we demonstrated the feasibility of a novel MRI technique for compounds with complex spectra, and demonstrated its high sensitivity both in vitro and in vivo.
Acknowledgements
This work was supported by grants from the Pierre Mercier Foundation, the Swiss Multiple Sclerosis Society and the Swiss National Science Foundation (PZ00P3-154719) to RBvH, as well as by the Centre d’Imagerie BioMédicale (CIBM) of the UNIL, UNIGE, HUG, CHUV, EPFL, and the Leenaards and Jeantet Foundations.References
1. Ruiz-Cabello J, Barnett BP, Bottomley PA, Bulte JW. Fluorine (19F) MRS and MRI in biomedicine. NMR Biomed 2011; 24(2): 114–129.
2. Ahrens ET, Zhong J. In vivo MRI cell tracking using perfluorocarbon probes and fluorine-19 detection. NMR Biomed 2013;26(7):860-71.
3. Ahrens ET, Helfer BM, O'Hanlon CF, Schirda C. Clinical cell therapy imaging using a perfluorocarbon tracer and fluorine-19 MRI. Magn Reson Med 2014;72(6):1696-701.
4. Riess JG. Oxygen Carriers (“Blood Substitutes”) - Raison d’Etre, Chemistry, and Some Physiology. Chem Rev 2001, 101, 2797-2919
5. Brodsky EK, Holmes JH, Yu H, Reeder SB. Generalized k-space decomposition with chemical shift correction for non-Cartesian water-fat imaging. Magn Reson Med 2008;59(5):1151-64
6. Goette MJ, Keupp J, Rahmer J et al. Balanced UTE-SSFP for 19F MR imaging of complex spectra. Magn Reson Med 2015;74(2):537-43
7. Jacoby C, Oerther T, Temme S, et al. Simultaneous MR imaging at different resonance frequencies using multi chemical shift selective (MCSS) RARE. Proc Int Soc Magn Reson Med 2014; 2927
8. Colotti R, Bastiaansen JA, Wilson A, et al. Characterization of perfluorocarbon relaxation times and their influence on the optimization of fluorine-19 MRI at 3 Tesla. Magn Reson Med 2016, in press
Figures