0487
Using Hyperpolarized 129Xe MRI to Detect Impaired Cerebral Perfusion in Human Brain with Alzheimer’s Disease1Department of Chemistry, Lakehead University, Thunder Bay, ON, Canada, 2Thunder Bay Regional Health Research Institute, Thunder Bay, ON, Canada, 3Thunder Bay Regional Health Sciences Centre, Thunder Bay, ON, Canada, 4Northern Ontario School of Medicine, Thunder Bay, ON, Canada
Synopsis
In this work, we present the first hyperpolarized 129Xe human brain MR spectra and human brain MR images from participants with Alzheimer’s Disease. We found a marked difference in the wash-out of dissolved phase xenon from the brain between the two groups, likely resulting from impaired cerebral blood flow in the Alzheimer’s Disease participants. By exploring this difference, we demonstrate the feasibility and sensitivity of hyperpolarized 129Xe MRI in detecting changes in cerebral perfusion and evaluating this important physiological characteristic during an early stage of mild to moderate Alzheimer’s Disease.
Purpose
Hyperpolarized (HP) xenon-129 (129Xe) MRI has long been used to study structural and functional information of the lungs. Because inhaled xenon dissolves in the blood and is carried to brain tissue by cerebral blood flow (CBF), this emerging technique is a powerful tool to probe cerebral physiological information 1,2. Alzheimer’s Disease (AD) is a neurodegenerative disease with characteristics that include reduced CBF which starts at an early predementia stage 3. The aim of this work was to use HP 129Xe MRI to probe and compare the cerebral perfusion between healthy participants and those with AD.Methods
All procedures were reviewed and approved by local institutional Research Ethics Boards and all participants provided written informed consent. HP 129Xe brain MRS and MRI scans were performed dynamically in three participants with diagnosed mild to moderate AD (ages 72 ± 7 years, Montreal Cognitive Assessment (MoCA) score 21 ± 3), and five healthy older adults (age 64 ± 7 years, MoCA score 28 ± 1). All scans were performed on a Philips Achieva 3T clinical MR scanner, using a 1H/129Xe dual-tuned, dual-quadrature head coil (Clinical MR Solutions LLC, Brookfield, WI, USA). Isotopically enriched (84%) 129Xe gas was polarized to 30% using a commercial SEOP polarizer (Xemed LLC, Durham, NH, USA). 500mL (for spectroscopy) and 1L (for imaging) of xenon gas was delivered to the participant prior to each scan, followed by a 20s-breathhold. The procedure was well tolerated by all healthy and AD participants.
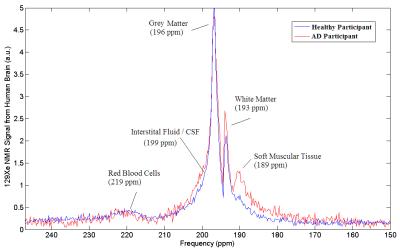
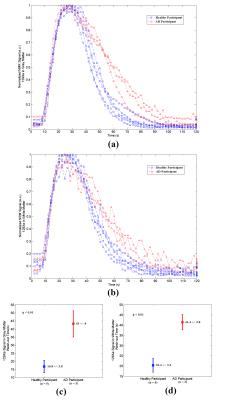
MR spectra were acquired immediately after gas inhalation using the following parameters: pulse-acquire sequence, centred at 196ppm from the xenon gas peak (referenced to 0ppm), 60 dynamics, TR = 2s, flip angle = 10o, bandwidth = 906ppm, spectral resolution = 0.22ppm, and a total scan time of 2 minutes. The time courses of 129Xe signals dissolved in grey matter (at 196ppm) and white matter (at 193ppm) were extracted from the acquired spectra. The wash-out time of 129Xe was derived by fitting the data from its maximum signal time point until the last time point, using previously employed models 4.
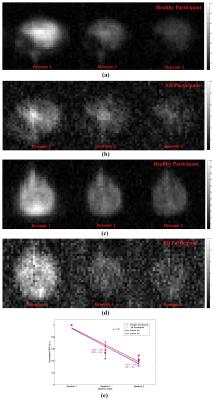
In addition, MRI scans were dynamically acquired at 10s, 20s, and 30s from the start of a 20s-breathhold after an additional inhalation of xenon gas, using the following parameters: 2D Fast Field Echo (FFE) sequence, sagittal/axial view, FOV = 250mm x 250mm, matrix size = 32 x 32, TR/TE = 250ms/0.84ms, flip angle = 12o, receiver bandwidth = 150Hz/pixel, NSA = 1. The SNR of the second and third dynamic images were normalized to that of the first dynamic image. The decline in SNR across the three dynamic images was calculated and compared between healthy and AD participants.
A two-tailed Student T-test was used to assess the statistical significance of differences in the wash-out values between the two groups in both MR spectra and MRI studies.
Results & Discussion
Four of five previously identified xenon peaks 5 were well resolved in 129Xe spectra from the brain of both healthy and AD participants, whilst the peak from xenon in interstitial fluid / CSF (at 199ppm) is barely observable in the healthy group and not resolved in the AD group (possibly due to a lower SNR). Xenon wash-out times in both grey and white matter, measured from the time series spectra, are statistically significantly longer (p < 0.01) in individuals with AD than in the healthy controls (Figure 2c and 2d). From the dynamic images, the decline in SNR of the three dynamics images shows a slower trend in the AD subjects compared to healthy controls (Figure 3e), which also suggests a slower xenon wash-out time. Both the spectroscopy and imaging studies in this work suggest a slower wash-out of xenon signal in the AD subjects. We hypothesize that these signal differences result from an impaired cerebral perfusion.Concusion
To our knowledge, this is the first work to present HP 129Xe MR spectra and images from the human brain with AD, and to use this technique to investigate physiological changes in human brain caused by Alzheimer’s Disease. We have demonstrated statistically significant different wash-out times of 129Xe from the brain of healthy and AD participants using spectroscopy, and observed a similar trend in the imaging studies. These preliminary results suggest that HP 129Xe MRI is potentially a sensitive tool to probe changes in cerebral perfusion caused by Alzheimer’s Disease at an early predementia stage, and possibly for other neurodegenerative diseases as well.Acknowledgements
This work was funded by Weston Brain Foundation, and partially supported by Thunder Bay Regional Health Research Institute and Lakehead University. The views expressed in this abstract are those of the authors and not necessarily those of Weston Brain Foundation, Thunder Bay Regional Health Research Institute or Lakehead University.References
1. Mazzanti ML, Walvick RP, Zhou X, et al.: Distribution of Hyperpolarized Xenon in the Brain Following Sensory Stimulation: Preliminary MRI Findings. PLoS One 2011; 6:e21607.
2. Madhwesha Rao, Neil J Stewart, Graham Norquay, Paul D Griffiths, and Jim M Wild, Proc. Intl. Soc. Mag. Reson. Med. 24 (2016), 0722
3. Binnewijzend MA, Benedictus MR, Kuijer JP, van der Flier WM, Teunissen CE, Prins ND, Wattjes MP, van Berckel BN, Scheltens P, Barkhof F. Cerebral perfusion in the predementia stages of Alzheimer's disease. Eur Radiol. 2016 Feb;26(2):506-14. doi: 10.1007/s00330-015-3834-9. Epub 2015 Jun 5.
4. Kilian W, Seifert F, Rinneberg H., Dynamic NMR spectroscopy of hyperpolarized (129)Xe in human brain analyzed by an uptake model. Magn Reson Med. 2004 Apr;51(4):843-7.
5. Rao M, Stewart NJ, Norquay G, Griffiths PD, Wild JM: High resolution spectroscopy and chemical shift imaging of hyperpolarized 129 Xe dissolved in the human brain in vivo at 1.5 tesla. Magn Reson Med 2016; 75:2227–2234.
Figures