0485
Specific Absorption Rate, dB/dt, and Device Parameters Following MRI of Patients with Implanted Cardiac Devices1Johns Hopkins University, Baltimore, MD, United States, 2Siemens Medical Solutions
Synopsis
This large prospective study showed that the previously suggested SAR limit of 2 W/kg can be unduly restrictive. No other MRI characteristics appear to predict changes in device system parameters.
Introduction
Magnetic resonance imaging (MRI) is considered as the modality of choice in many clinical situations. Unfortunately, MRI is currently unavailable for a large number of patients who have implanted cardiac devices such as pacemakers, implantable cardioverter defibrillators (ICD) and cardiac resynchronization therapy (CRT) devices. Associated risks with performing MRI arise from the interaction between the implanted device system and three essential components of MRI which are the static and gradient magnetic fields, and radiofrequency (RF) magnetic field. There have been tremendous efforts to develop MRI safety protocols to reduce complications in patients with cardiac devices (1-3). Radiofrequency energy absorption may lead to heating in biologic tissue. Moreover, presence of conductive implants in the body raises the concern of RF heating in leads and tissues surrounding such devices. Thermal injury and the subsequent formation of fibrosis may result in increasing capture thresholds, and deterioration of device function. Specific absorption rate (SAR) and specific energy dose (SED) are the dosimetric terms used to characterize the thermogenic aspects of the electromagnetic field. In study we aimed to explore the effect of MRI field components (gradients and RF magnetic fields), region of imaging, and cardiac device characteristics to determine which of these factors has detrimental effects on device parameters.Methods
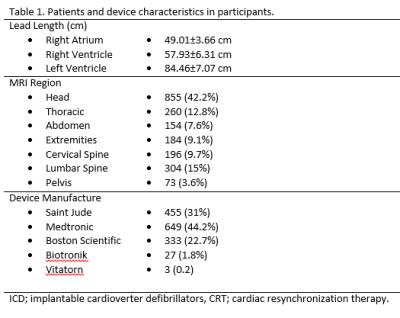
1,467 patients [age: 66.71±14.98yrs, Male: 930 (63.4%)] who were referred to undergo medically necessary MRI were prospectively enrolled in this study. A total of 2,027 MRI examinations were performed in these patients with a total 3,832 leads using clinical MRI protocols without SAR restrictions. To evaluate appropriate device function atrial and ventricular sensing, lead impedance, and capture threshold were measured before and immediately after MRI examination. Two experienced investigators evaluated the device and lead variables. Imaging was performed at 1.5 Tesla using an Avanto scanner (Siemens, Erlangen, Germany). Magnetic resonance imaging was performed according to standard institutional protocols for the region of interest using usual protocols with standard peak SAR settings for the scan. Whole body averaged SAR was reported based upon the MR sequence parameters, applied voltages, the transmitter reference adjustment and the patient weight. The data regarding the gradient slew rate for y-gradient, x-gradient and z-gradient coils in addition to the scan duration time for each sequence were recorded from the scanner.Results
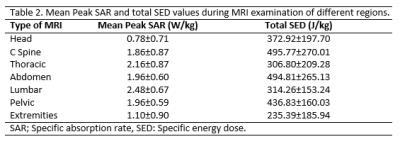
876 patients (59.71%) had a permanent pacemaker, 436 (29.72%) had an ICD, and 155 (10.56%) had a CRT system. Among the patients, 123 (8.4%) were device dependent. Lead length, MRI regions and device manufacturers have been summarized in table 1. The average number of acquired sequences during each scan was 18±12.6. The values for peak SAR, peak SED, during MRI scan of different regions have been summarized in table 2. Following the previously described safety protocol, no adverse events including device movement, torque, heating, arrhythmia, or unexpected device activation were reported during the scans and all devices were functioning properly following scan. Comparison of device interrogation findings obtained before, immediately and 6 months after MRI showed no significant changes in battery voltage, lead thresholds, lead impedances, or sensing signal amplitudes. In multivariable analysis, no associations between changes in device and lead parameters and scanned region, scan duration, SAR or dB/dt. However, changes in device right ventricular (RV) sensing and capture thresholds were associated with RV lead length (P<0.05).Discussion
To the best of our knowledge, this is the first prospective study to evaluate the association between cardiac device parameters and MRI field and implantable device characteristics. The main finding of the current study is that MRI characteristics including dB/dt, SAR, SED, and scan duration were not associated with adverse device changes. These findings are crucial particularly in patients with implanted legacy devices, as the need to scan them will continue for many years. The current results show that the previously suggested SAR limit of 2 W/kg can be unduly restrictive. Additionally, no other MRI characteristics appear to predict changes in device system parameters. However, the association of lead length with parameter changes warrants further study.Acknowledgements
The study was funded by NIH grants K23HL089333 and R01HL116280 to Dr. Nazarian.References
(1) Nazarian S, et al. Clinical utility and safety of a protocol for noncardiac and cardiac magnetic resonance imaging of patients with permanent pacemakers and implantable-cardioverter defibrillators at 1.5 tesla. Circulation 2006 Sep 19;114(12):1277-84.
(2) Nazarian S, et al. A prospective evaluation of a protocol for magnetic resonance imaging of patients with implanted cardiac devices. Ann Intern Med 2011 Oct 4;155(7):415-24.
(3) Roguin A, et al. Modern pacemaker and implantable cardioverter/defibrillator systems can be magnetic resonance imaging safe: in vitro and in vivo assessment of safety and function at 1.5 T. Circulation 2004 Aug 3;110(5):475-82.