0479
A personalised SAR model for subject-specific RF safety1Nuffield Department of Clinical Neurosciences, FMRIB Centre, University of Oxford, Oxford, United Kingdom, 2Department of Cardiovascular Medicine, Department of Cardiovascular Medicine, University of Oxford, Oxford, United Kingdom
Synopsis
In this study, we introduce a method to personalise SAR modelling by non-linear registration of a high-resolution reference voxel model into a target (subject-specific) head morphometry. We evaluate this by using two well characterised electromagnetic models, Duke and MIDA, by comparing MIDA-warped-into-Duke (MIKE), Duke and MIDA. Maps of 10g SAR across a range of B1+ shims were evaluated, showing improved agreement between the MIKE and Duke models, versus the native MIDA and Duke models. By employing personalised SAR models an increased confidence in EM simulation can be achieved.
Purpose
Electromagnetic (EM) simulations are used to assess radiofrequency (RF) heating in MRI. However, few studies have accounted for anatomical differences between individuals. This is relevant for parallel transmit (pTx), which has the potential to actively reduce SAR, provided there is confidence in the model of energy deposition1,2. Those studies that have demonstrated a personalised voxel model have used segmented MR images, which struggle to represent all required tissue types, and usually lack sufficient spatial resolution. In this study, we introduce a method of personalised SAR modelling using a high-resolution reference voxel model that includes full EM characterization. We hypothesise that this will provide a closer agreement to the true SAR deposition than a generic model alone.Methods
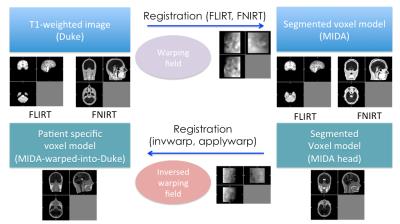
The Duke (Male, 34yrs) and MIDA (Female, 29yrs) human simulation models (IT’IS Foundation, Switzerland) were used to validate a method of non-linear registration of a high-resolution voxel model into a personalised electromagnetic model3,4. The Duke whole-body voxel model was cut at the level of the T5 vertebrae to match to the size of the MIDA model. Simulated MRI tissue intensities were taken from T1-weighted images acquired separately using a 3T Siemens PRISMA MRI scanner (Siemens, Germany) using a 64-channel head and neck coil with T1-MPRAGE (TR/TE 2380/3.96 ms, TI 1200 ms, FA 8°). T1 intensities were imported to both Duke and MIDA voxel models. Tools from the FMRIB Software Library (FSL) were used to perform registration of the voxel models5 (Fig. 1). The MIDA model was down-sampled from 0.5 mm3 to 2 mm3 to avoid over-fitting of fine structures and to provide better agreement of warping across larger areas. Warping was accomplished by:
· a 6-degrees-of-freedom affine registration to locate the MIDA model into the same overall space as the Duke model, via an MNI 152 template, using the FLIRT (FMRIB's Linear Image Registration Tool), with brain extracted images6.
· non-linear registration on whole-head data was performed by minimising the least-squares difference using FNIRT (FMRIB's Nonlinear Image Registration Tool).
· applying an inverse warping field to the MIDA model to warp it into Duke space.
· attaching the torso of the Duke model to both the MIDA and the MIDA-warped-into-Duke (MIKE) model to account for energy absorption in the shoulder regions.
Sim4Life (ZMT, Switzerland) was used to calculate the B1 field and SAR distribution, and a model of an 8-channel transceiver array coil (Affinity Imaging GmbH, Juelich, Germany) was incorporated. When simulating circularly polarised (CP) mode, the B1+ field, SAR and 10g averaged SAR were normalized to fields that produced 2μT at the coil centre using ideal current driving method7,8. To simulate arbitrary pTx modes, the capacitances of the coil model were tuned to the load of the Duke whole body model. And eight separate simulations were performed by adding 50 ohm resistances to the non-activated channels and normalising to 1W conducted power for each channel, which were then used to calculate a Q-matrix9. For local SAR calculation the Q-matrix was averaged over a 10g mass10,11. To assess maximum 10gSAR the averaged Q-matrix was used to assess 5,000 B1+ shims with random amplitude (0-1) phase (0-2π).
Results
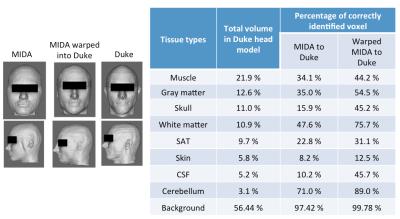
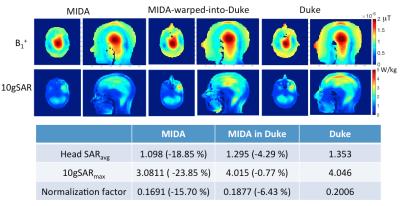
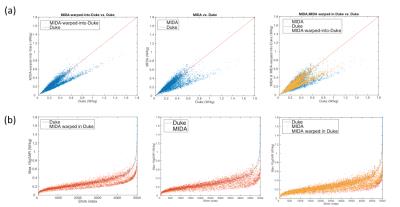
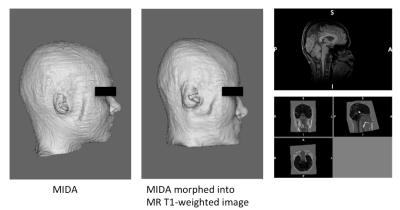
The MIKE model was compared with the native Duke and MIDA models by counting correctly identified voxels (Fig. 2). The B1+ field distribution and 10g averaged SAR in CP mode are shown in Fig. 3. In CP mode the MIKE and Duke models showed close agreement (< 10% difference) for head averaged SAR and 10g maximum SAR. In pTx mode the maximum 10g SAR for each of 5000 shim settings are plotted in Fig. 4. The largest difference in 10g SAR between Duke and MIDA was 50%, while for MIKE it was reduced to a 38.4% difference. To evaluate real-life applicability the MIDA model was successfully warped into a T1w scan acquired in a volunteer, demonstrating the possibility of a personalised high-resolution voxel model (Fig. 5).Discussion and Conclusions
A method to achieve personalized SAR modelling has been evaluated and shows close agreement in CP mode. For local SAR in pTx the predicted SAR in warped MIDA tended towards that of Duke, although there is still room for improvement. As Fig. 2 shows, improvements can be made to the warping process, which can be achieved by fine tuning the synthetic T1-intensities of MIDA’s 135 tissue types and by warping a CAD model rather than a voxel model to provide better continuity of tissue. In conclusion, the personalised data set (MIKE) provided close agreement to the target ‘patient’ dataset (Duke) and as a result provides increased confidence in personalized EM simulation.Acknowledgements
Oxford-Radcliffe Graduate Scholarship (University College Oxford) and Clarendon Fund.References
1. Katscher U, Bö P, Leussler C, Van Den Brink JS. Transmit SENSE. Magn Reson Med. 2003. doi:10.1002/mrm.10353.
2. Wu X, Akgün C, Vaughan JT, et al. Adapted RF Pulse Design for SAR Reduction in Parallel Excitation with Experimental Verification at 9.4 Tesla.
3. Christ A, Kainz W, Hahn EG, et al. The Virtual Family—development of surface-based anatomical models of two adults and two children for dosimetric simulations. Phys Med Biol. 2010;55:23-38. doi:10.1088/0031-9155/55/2/N01.
4. Iacono MI, Neufeld E, Akinnagbe E, et al. MIDA: A Multimodal Imaging-Based Detailed Anatomical Model of the Human Head and Neck. PLoS One. 2015;10(4). doi:10.1371/journal.pone.0124126.
5. Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. In: NeuroImage.; 2004. doi:10.1016/j.neuroimage.2004.07.051.
6. Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001. doi:10.1016/S1361-8415(01)00036-6.
7. Collins CM, Smith MB. Calculations of B1 distribution, SNR, and SAR for a surface coil adjacent to an anatomically-accurate human body model. Magn Reson Med. 2001. doi:10.1002/mrm.1092.
8. Jeong H, Papoutsis K, Jezzard P, Hess AT. Faster B1 Field and SAR Estimation in Parallel Transmit Arrays without Tuning using Voltage Sources. In: Proc. Intl. Soc. Mag. Reson. Med. Toronto; 2015.
9. Graesslin1 I, Wang2 S, Biederer3 S, et al. Towards Patient-specific SAR Calculation for Parallel Transmission Systems. In: Proc. Intl. Soc. Mag. Reson. Med. .; 2010.
10. Carluccio G, Erricolo D, Oh S, Collins CM. An approach to rapid calculation of temperature change in tissue using spatial filters to approximate effects of thermal conduction. IEEE Trans Biomed Eng. 2013. doi:10.1109/TBME.2013.2241764.
11. Graesslin I, Homann H, Biederer S, et al. A Specific Absorption Rate Prediction Concept for Parallel Transmission MR. Magn Reson Med. 2012;68:1664-1674. doi:10.1002/mrm.24138.
Figures