0473
Optimal framework for quantitative magnetization transfer imaging of small structures1UCL Institute of Neurology, Queen Square MS Centre, UCL, London, United Kingdom, 2Centre for Medical Image Computing, Department of Computer Science, UCL, London, United Kingdom, 3Champalimaud Neuroscience Programme, Champalimaud Centre for the Unknown, Lisbon, Portugal, 4Philips Healthcare, Guilford, United Kingdom, 5Translational Imaging Group, Centre for Medical Image Computing, Department of Medical Physics and Biomedical Engineering, UCL, London, United Kingdom, 6Department of Medical Physics and Bioengineering, UCL, London, United Kingdom, 7CISC, Brighton & Sussex Medical School, Brighton, United Kingdom, 8Department of Brain and Behavioural Sciences, University of Pavia, Pavia, Italy, 9Brain MRI 3T Mondino Research Center, C. Mondino National Neurological Institute, Pavia, Italy
Synopsis
Quantitative Magnetization Transfer (qMT) Imaging allows quantification of parameters describing the macromolecular component of tissues, potentially specific for myelin in the central nervous system. To date, applications of qMT in small structures (e.g. the spinal cord) have been hampered by prohibitively long acquisition. We present a framework for robust qMT examinations in small structures. It consists of: a dedicated MT-weighted sequence for reduced field-of-view imaging, explicit modelling of the non-steady state signal, and optimal definition of the sampling scheme. Superiority of the framework compared to a conventional qMT protocol is demonstrated in the healthy spinal cord and in the brainstem.
Purpose
Quantitative Magnetization Transfer (qMT) methods allow the conventionally inaccessible macromolecular component of tissues to be probed. In particular, qMT provides quantification of parameters such as the macromolecular fraction (BPF), macromolecular T2 (T2B), free water T2 (T2F), and free water to macromolecules exchange rate (kFB), which have proven valuable in assessing myelin integrity in the central nervous system1.
However, translation of qMT from the brain to small, yet key, structures, such as the spinal cord (SC), a primary location in demyelinating diseases, has not been fully implemented2,3, as demands of high-resolution and adequate SNR result in prohibitive protocol lengths.
We propose a framework to enable robust assessment of qMT parameters in the SC within a clinically feasible scan time. An MT-weighted reduced Field-of-View echo-planar imaging (EPI) sequence is combined with a dedicated model for unbiased parameter estimation. The sampling scheme is optimized via Cramer-Rao-Lower-Bounds (CRLBs) minimization4. Reproducibility of qMT metrics is shown in the healthy SC, and the versatility of the framework for investigating other small structures is demonstrated in the brainstem, crucial for several neurodegenerative diseases and often characterized through MT imaging5.
Methods
Image acquisition is performed with ZOOM-EPI6. MT sensitization is achieved via off-resonance pulse trains preceding slice excitations. A numerical model based on the coupled Bloch equations7 is used for parameter estimation, to account for the non-steady-state nature of the acquisition. In addition to pulse amplitude (B1) and offset frequency (Δ), effects of pulse duration (τ), inter-pulse gap (Δt), and number of pulses (N) are also modelled.
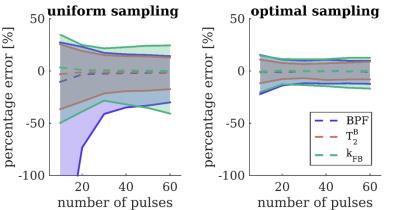
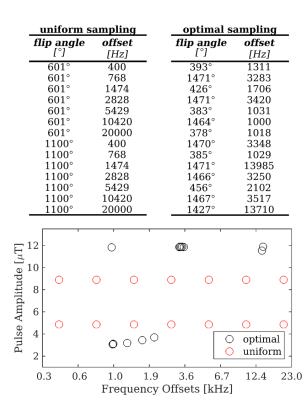
CRLB theory is used to derive configurations of (B1,Δ,τ,Δt,N) that maximise precision of (BPF,T2F,T2B,kFB), for a fixed number of data-points K=14 (+1 reference image), and under realistic SAR constraints. CRLBs are minimized using a self-organizing migratory algorithm (SOMA)8, to produce sets of optimal pairs (Δ,B1). Remaining parameters (τ,Δt,N) are selected by comparing a posteriori cost function values for optimisations at several combinations of (τ,Δt,N). The cost function is given by the weighted mean of BPF, T2B and kFB CRLBs.
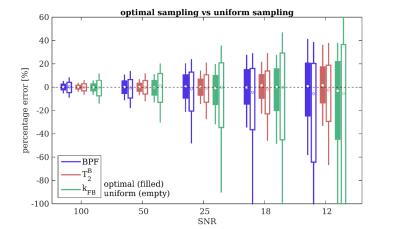
The efficacy of optimization is tested using Monte-Carlo simulations (N=1000 repetitions of Rician noise at SNR=100,50,25,18,12), comparing parameter estimate errors from the optimal protocol against a scheme based on standard steady-state qMT acquisition (i.e. uniform sampling)9.
Reproducibility indices (I) of qMT metrics, from a repeated acquisition (three times) on the same subject, are compared between optimal and uniform sampling. For a given parameter p, I(p) is defined as $$$1-\frac{1}{2}(\frac{max(p)-min(p)}{mean(p)})$$$, where I(p)=1 means ideal reproducibility. Qualitative comparison of sampling schemes is performed in the brainstem to highlight the flexibility of the proposed framework.
In vivo imaging is performed on a 3T Philips Achieva system. The full protocol comprises 2 qMT acquisitions (optimal 21:27min, uniform 23:44min), and a shared Inversion-Recovery sequence (8 TIs, TImin/Δt=150ms/350ms) for T1 estimation (15:06min); 12 axial slices are acquired at 0.75x0.75x5mm3 resolution in the SC, or at 0.9x0.9x3mm3 in the brainstem.
Results
Figure 1 shows optimised protocols producing low estimation errors and having little dependence on N by taking SAR and B1 limits into account in the optimisation. For N=25, the best configuration for (τ,Δt) was found heuristically at τ/Δt=15ms/15ms. Comparison of optimal (Δ,B1) sampling at (τ/Δt/N=15ms/15ms/25) and uniform sampling for simulations and in vivo are given in figure 2.
Monte-Carlo simulations demonstrate the superiority of optimal compared to uniform sampling, consistent at different SNR levels (figure 3).
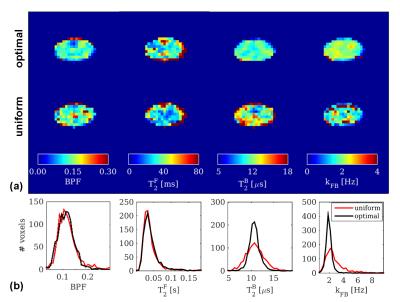
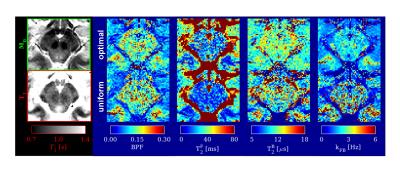
In vivo, qMT parameters in the SC and brainstem (figures 4,5) show reduced variability when obtained from optimal sampling. The improvement over uniform sampling is particularly evident for kFB. Reproducibility indices (uniform/optimal) in the SC are: I(BPF)=0.74/0.74, I(T2F)=0.66/0.62, I(T2B)=0.83/0.87, I(kFB)=0.58/0.82.
Discussion
We combine explicit modelling of the non-steady-state MT signal and protocol optimization to simultaneously improve parameter estimates and shorten scan time compared to conventional sampling.
kFB is the parameter that benefits the most from optimisation, given its large intrinsic variability as previously reported10. Reproducibility index did not show improvements in BPF, therefore further investigations are needed to clarify this aspect, including specific protocol optimisations targeting only BPF.
Our framework allows to choose pulse train duty-cycle and duration independently, which we exploit for shorter and more efficient protocols. Here, optimal configuration for (τ,Δt,N) was found empirically, which could lead to suboptimal protocols. The full set of sequence parameters will be optimized simultaneously in future analysis.
The proposed framework is versatile and can be used for any type of localized qMT examination. We have demonstrated its applicability to the brainstem, to delineate differences between the substantia nigra and the surrounding white matter, and provide new complementary information towards the characterization of this structure using MT11.
Acknowledgements
The UK MS Society. NIHR BRC UCLH/UCL High Impact Initiative (BW.mn.BRC10269). Horizon2020-EU3.1 CDS-QUAMRI (ref: 634541). Project grants ISRT (IMG006), EPSRC (EP/I027084/1, EP/H046410/1, EP/J020990/1, EP/K005278), and MRC (MR/J500422/1, MR/J01107X/1).References
[1] Sled J, Pike B. Quantitative imaging of magnetization transfer exchange and relaxation properties in vivo using MRI. Magnetic Resonance in Medicine 46.5(2001):923-931. [2] Smith S, Golay X, Fatemi A, et al. Quantitative magnetization transfer characteristics of the human cervical spinal cord in vivo: application to adrenomyeloneuropathy. Magnetic Resonance in Medicine 61.1(2009):22-27. [3] Smith A, Dortch R, Dethrage L, et al. Rapid, high-resolution quantitative magnetization transfer MRI of the human spinal cord. NeuroImage 95(2014):106-116. [4] Atkinson A, Donev A. Optimum experimental design. Oxford: Oxford University Press (1992). [5] Tambasco N, Nigro P, Romoli M, et al. Magnetization transfer MRI in dementia disorders, Huntington's disease and parkinsonism. Journal of the neurological sciences 353.1(2015):1-8. [6] Wheeler-Kingshott C, Hickman S, Parker G, et al. Investigating cervical spinal cord structure using axial diffusion tensor imaging. NeuroImage 16.1(2002):93-102. [7] Portnoy S, Stanisz G. Modeling pulsed magnetization transfer. Magnetic Resonance in Medicine 58.1(2007):144-155. [8] Alexander D. A general framework for experiment design in diffusion MRI and its application in measuring direct tissue-microstructure features. Magnetic Resonance in Medicine 60.2(2008):439-448. [9] Cercignani M, Symms M, Schmierer K, et al. Three-dimensional quantitative magnetisation transfer imaging of the human brain. NeuroImage 27.2(2005):436-441. [10] Cercignani M, Alexander D. Optimal acquisition schemes for in vivo quantitative magnetization transfer MRI. Magnetic Resonance in Medicine 56.4(2006):803-810. [11] Helms G, Draganski B, Frakowiak R, et al. Improved segmentation of deep brain grey matter structures using magnetization transfer (MT) parameter maps. NeuroImage 47.1(2009):194-198.Figures