0469
More Than Simply Iron: How the Mesoscopic and Cellular Distribution of Iron Impacts the MR Contrast1Neurophysics, Max Planck Institute for Human Cognitive and Brain Sciences, Leipzig, Germany, 2Center for Cognitive Neuroscience Berlin, Free University Berlin, Berlin, Germany, 3Paul-Flechsig-Institute for Brain Research, Leipzig, Germany, 4Medical Physics Group, Institute of Diagnostic and Interventional Radiology, Jena University Hospital – Friedrich Schiller University Jena, Jena, Germany, 5Section of Experimental Neurology, Department of Neurology, Essen University Hospital, 6Department of Solid State Physics, Faculty of Physics and Earth Sciences Leipzig University, Leipzig, 7Bundesanstalt für Materialforschung und –prüfung BAM, Berlin, Germany
Synopsis
Iron is an important source of MRI contrast in the brain. Herein, we investigated the influence of the cellular and subcellular iron distribution on the iron-induced MR contrast. Quantitative MRI on post mortem brain samples was combined with quantitative iron mapping and numerical simulations of local field distributions. We show that iron is heterogeneously distributed in both grey and white matter as well as in subcortical nuclei and different scales of heterogeneity play a role for MR contrast in these regions. Our results provide an important step towards quantitative understanding of iron induced MR-contrast and its microstructural underpinnings.
PURPOSE
Iron is a major source of MR contrast in the brain. It dominates variation of transverse (R2) and effective transverse (R2*) relaxation rate as well as quantitative susceptibility maps (QSMs) across the cortex, contributes to white matter contrast and drives the differential contrast in brain nuclei and subcortical white matter1. A lot of theoretical2 and experimental studies focused on iron-induced MR-contrasts3–8 aiming at applications such as in-vivo Brodmann mapping and MR-based iron quantification. However, the influence of the exact cellular and subcellular iron distribution on MR contrast has been largely ignored so far. A majority of proposed models rely on a single set of linear coefficients to describe the relation between tissue iron concentration and relaxation rates or magnetic susceptibilities4,7,9. This is partly due to a lack of quantitative knowledge on the cellular and subcellular iron distribution in the human brain and the absence of dedicated quantitative histological methods. We address this issue by combining quantitative MRI with quantitative iron mapping on post mortem brain samples and numerical simulations of field distributions.METHODS
We investigated post-mortem human brain samples excised from the temporal lobe (male, 78y) and the midbrain (female, 71y). Multi-echo gradient echo (isotropic resolution of 0.21 mm and 0.06 mm) imaging was performed at 7 Tesla. R2* and susceptibility maps were computed using mono-exponential fitting of the GRE signal magnitude and susceptibility mapping based on GRE signal phase using the HEIDI algorithm, respectively. Tissue iron in one subsample of the temporal lobe was removed by incubating the sample in a deferroxamin solution. Turnbull and Perls’s iron staining of the tissue samples was carried out display the distribution of Fe2+ and Fe3+, respectively. Laser Ablation Inductively Coupled Mass Spectroscopic Imaging (LA-ICP-MSI) and Proton Induced X-ray Emission (PIXE) microscopy of the tissue samples provided quantitative iron maps with a mesoscopic resolution of 12 µm x 60 µm x 120 µm and a microscopic spatial resolution of 1 µm, respectively. Based on the microstructural iron distribution observed with PIXE microscopy we modelled iron induced magnetic field perturbations at a microscopic and mesoscopic scale in order to quantify the iron driven static de-phasing contribution in R2*. Furthermore, a Gaussian diffusion model was used to estimate the contribution of proton diffusion in the iron-induced magnetic field perturbations to R2 and R2* in grey matter, white matter and substantia nigra.RESULTS AND DISCUSSION
We show
that iron is heterogeneously distributed in both grey and white matter as well
as in subcortical nuclei and different scales of heterogeneity play a role for
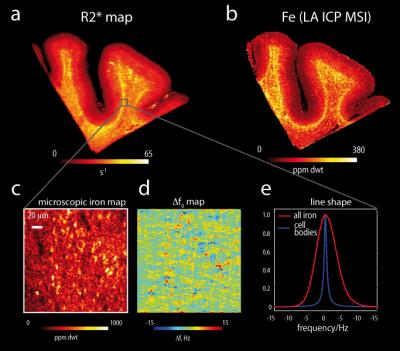
MR contrast in these regions. In the cortical grey matter, the iron
concentration increased from the pial surface towards the gray/white matter
boundary3 and it was also increased in layer IV, dominating both
the R2* and susceptibility contrasts in these areas (Fig.1). On the microscopic scale sparsely distributed
iron rich fibres, and small micro-, astro- and oligodendroglia substantially
contribute to R2* and susceptibility. In superficial and deep white matter mainly
oligodendrocytes and iron rich fibres constituted the iron rich cellular
compartments (Fig.2). At the mesoscopic level patches of enhanced iron
concentration around small vessels with the typical size of 0.1mm - 0.2mm
significantly increased R2* in white matter (Fig.3). Modelled proton line
broadening (see Figs.2 and 3) resulting from iron distribution in superficial
and deep white matter corresponded well to the obtained changes in relaxation
rate R2* induced by de-ironing (see Fig. 3e). Iron located in
the cell bodies induced exponential decay and thus Lorentzian line broadening,
while the myelin bound iron along the fibres lead to Gaussian line broadening
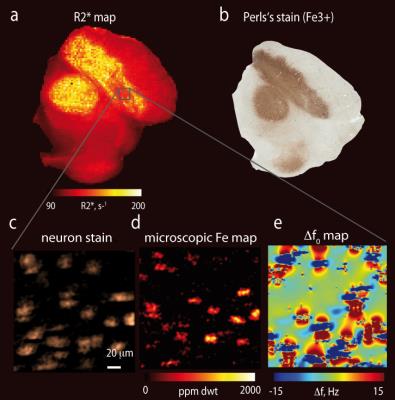
(see Fig.3f). Different characteristic iron distributions dominated the
contrast in the substantia nigra where high background iron level and densely
packed large iron loaded neurons were found (Fig. 4).CONCLUSIONS
We quantitatively characterized the mesoscopic and microscopic heterogeneity of iron distribution in grey and white matter as well as in the substantia nigra. We have shown that the gradient of iron concentration in the cortex drives the intracortical R2* MR contrast. Our results also indicate that not only myeloarchitechture but also microscopic iron distribution has to be taken into account in theoretical models to quantitatively describe white matter MR contrast and its anisotropy. In the substantia nigra our data may potentially be used as a first step towards mapping of density of dopaminergic neurons. Our results provide an important step towards quantitative understanding of iron induced MR-contrast and its microstructural underpinningsAcknowledgements
References
1. Kirilina E, et al. Imaging Subcortical White Matter by High Resolution 7 T MRI in vivo: Towards Potential U-Fiber Mapping in Humans. 24th Annual ISMRM Meeting, Singapore, 2016, #0502.
2. Yablonskiy D, et al. Biophysical mechanisms of MRI signal frequency contrast in multiple sclerosis. PNAS. 2012;109:14212-14217.
3. Fukunaga M, et al. Layer-specific variation of iron content in cerebral cortex as a source of MRI contrast. PNAS. 2010;107:3834–3839.
4. Stüber C, et al. Myelin and iron concentration in the human brain: A quantitative study of MRI contrast. Neuroimage. 2014;93(1):95–106.
5. Deistung A, et al. Toward in vivo histology: a comparison of quantitative susceptibility mapping (QSM) with magnitude-, phase-, and R2*-imaging at ultra-high magnetic field strength. Neuroimage. 2013;65:299–314.
6. Schweser F, et al. Quantitative susceptibility mapping for investigating subtle susceptibility variations in the human brain. Neuroimage.2012;62:2083–2100.
7. Langkammer C, et al. Quantitative susceptibility mapping (QSM) as a means to measure brain iron? A post mortem validation study. NeuroImage. 2012;62:1593–1599.
8. Langkammer C, et al. Quantitative MR Imaging of Brain Iron: A Postmortem Validation Study. Radiology. 2010;257:455–462.
9. Rooney W, et al. Magnetic field and tissue dependencies of human brain longitudinal 1H2O relaxation in vivo. Magn Reson Med. 2017;57:308–318.
Figures
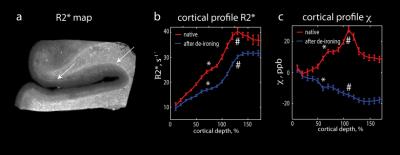
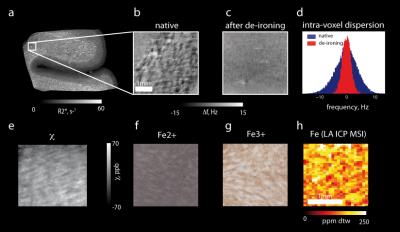
Figure 2. Iron induced contrast in white matter. (a) Quantitative R2* map. Frequency maps (b, c) and frequency spectrum (d) before and after de-ironing. (e) Susceptibility map before de-ironing. (f) Turnbull’s (Fe2+) iron stain and (g) Perls’s (Fe3+) iron stain. (h) Quantitative iron map measured with LA-ICP-MSI. Iron in white matter appears in patches with a characteristic size of 0.1-0.2 mmm (f, g, h), which disappear upon de-ironing (c). These patches localized around vessels induce substantial intra-voxel frequency dispersion and therefore contribute substantially to R2* in white matter.