0463
Functional response of corpus callosum to electrical stimulation of S1 cortex in mice1Radiology, Washington University School of Medicine, St. Louis, MO, United States, 2The Hope Center for Neurological Disorders, Washington University School of Medicine, St. Louis, MO, United States, 3Biostatistics, Washington University School of Medicine, St. Louis, United States, 4Neurology, The University of North Carolina in Chapel Hill, NC, United States, 5Neurology, Washington University School of Medicine, St. Louis, MO, United States, 6Biomedical Engineering, Washington University in St. Louis, St. Louis, MO, United States
Synopsis
The corpus callosum (CC) is the major pathway for interhemispheric communication and a primary target of white-matter neurodegenerative diseases. Diffusion MRI is widely used to assess white-matter structural alternations in diseases. In addition to morphological changes, we previously demonstrated the feasibility to assess white matter function using diffusion MRI. We observed a 27% perpendicular apparent diffusion coefficient (ADC⊥) decrease in normal mouse optic nerve during visual stimulation. In the current study, we implanted MR-compatible tungsten wires at primary somatosensory cortex and observed 15 – 21% ADC⊥ decrease in CC, suggesting diffusion MRI can be applied to study function at this site.
Introduction
Corpus callosum (CC) is a major white matter bundle for interhemispheric communication1 and commonly affected in a wide range of neurodegenerative diseases including, but not limited to, Parkinsons Disease, Alzheimer’s Disease, Huntington’s Disease and multiple sclerosis.2, 3 Diffusion MRI is widely used to assess white-matter pathologies. In addition to pathology-associated morphological changes, we previously demonstrated the feasibility to assess white matter function using diffusion MRI. We observed a 27% perpendicular apparent diffusion coefficient (ADC⊥) decrease (vs. baseline ADC⊥) in mouse optic nerve during visual stimulation.4 Such stimulation-associated ADC⊥ decrease was independent of vascular effects.4 In the current study, we implanted MR-compatible tungsten electrodes5 in the primary somatosensory cortex (S1). We performed diffusion fMRI measurement to assess the response of CC to demonstrate the feasibility of combining white-matter diffusion fMRI with electrical stimulation at S1, and to determine whether results in CC were in accord with optic nerve diffusion fMRI findings.Materials and Methods
Electrode implantation: A 15-mm midline skin incision was made and the skull exposed on 9-10 weeks old C57BL/6 female mice. A 50-µm diameter tungsten electrode was implanted through a 2-mm burr hole at right S1 barrel field: 1.2 mm posterior to the bregma, 3.0 mm lateral to the midline, 0.7 mm deep (Fig. 1 B). Stimulation conditions: Electrical stimulation was performed with biphasic square-waves of 6 Vpeak-peak, 12.5-pulse width at 40 Hz (n=7) or 6 Vpeak-peak, 8.3-ms pulse width at 60 Hz (n=5), respectively. DWI⊥: Mice were anesthetized with 0.8 - 1% isoflurane in O2. Diffusion MRI was performed using a 4.7-T Agilent small-animal MR scanner. A mid-sagittal slice was acquired to cover the entire CC using a multiple-echo spin-echo imaging sequence6 with the following parameters: TR = 1.5 s, TE = 34.4 ms, inter-echo delay = 18.2 ms, FOV = 20 × 20 mm2, matrix size = 256 × 256 (zero-filled to 512 × 512), and thickness = 1.0 mm. A pair of diffusion-weighted (DW) images with phase-encoding diffusion weighting (perpendicular to CC fibers) was acquired with b-value = 0.1 and 1.4 ms/µm2, δ = 5 ms, and Δ = 18 ms. Acquisition time was 12.8 minutes for each pair of DW images.4, 7 Diffusion fMRI strategy: We acquired a pair of DW images as baseline without stimulation, followed by a pair of DW images with electrical stimulation at S1. After a single stimulation session, a final pair of DW images was acquired immediately after the stimulation was turned off. Data analysis: ADC⊥ maps were generated from two DW images. ROI was drawn manually encompassing the CC on DWI with b = 1.4 ms/µm2.Results
The MR-compatible tungsten electrode was implanted at the right S1 cortex (Fig. 1 A and B). The mid-sagittal image slice was relatively perpendicular to CC fibers. With a pair of DW images with diffusion gradient in phase-encoding direction, the ADC⊥ of CC was quite homogeneous, suggesting minimal partial volume effect at CC with the current imaging protocol (Fig. 1 E). Representative ADC⊥ maps of CC at baseline before stimulation, during 40 or 60 Hz electrical stimulation, and after stimulation was turned off (Fig. 2) demonstrated that a stimulation-associated ADC⊥ decrease was observed which was reversible, returning towards its baseline value. A 21% and 15% ADC⊥ decrease was observed with 40 and 60 Hz stimulation, respectively, of a similar magnitude to the 27% relative reduction seen in the optic nerve in prior studies (Fig. 3).Conclusion
In the present study, we demonstrate the feasibility of diffusion fMRI with electrical stimulation under isoflurane anesthesia to assess axonal activation in mouse CC. The relative extent of ADC⊥ change was greater at lower stimulation frequency. A lower stimulating frequency also might induce a greater optic nerve decremental response, but larger numbers of animals must be studied to make firm conclusions on the stimulation frequency dependence of the decrement. Multiple variables may change the extent of nerve activation on ΔADC⊥.Acknowledgements
Authors thank Dr. SunHo Lee's experience sharing of tungsten-electrode fabrication. Supported in part by NIH R01-NS047592, P01-NS059560, U01-EY025500 and NMSS RG 5258-A-5.References
1. Wang CL, Zhang L, Zhou Y, et al. Activity-dependent development of callosal projections in the somatosensory cortex. J Neurosci 2007;27:11334-11342.
2. Wiltshire K, Foster S, Kaye JA, Small BJ, Camicioli R. Corpus callosum in neurodegenerative diseases: findings in Parkinson's disease. Dementia and geriatric cognitive disorders 2005;20:345-351.
3. Rosas HD, Lee SY, Bender AC, et al. Altered white matter microstructure in the corpus callosum in Huntington's disease: implications for cortical "disconnection". NeuroImage 2010;49:2995-3004.
4. Spees WM, Lin TH, Song SK. White-matter diffusion fMRI of mouse optic nerve. NeuroImage 2013;65:209-215.
5. Lai HY, Albaugh DL, Kao YC, Younce JR, Shih YY. Robust deep brain stimulation functional MRI procedures in rats and mice using an MR-compatible tungsten microwire electrode. Magnetic resonance in medicine 2015;73:1246-1251.
6. Tu TW, Budde MD, Xie M, et al. Phase-aligned multiple spin-echo averaging: a simple way to improve signal-to-noise ratio of in vivo mouse spinal cord diffusion tensor image. Magnetic resonance imaging 2014;32:1335-1343.
7. Lin TH, Spees WM, Chiang CW, Trinkaus K, Cross AH, Song SK. Diffusion fMRI detects white-matter dysfunction in mice with acute optic neuritis. Neurobiology of disease 2014;67:1-8.
Figures
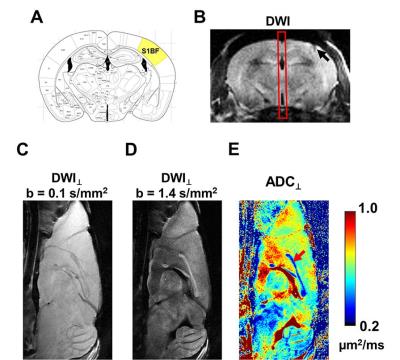
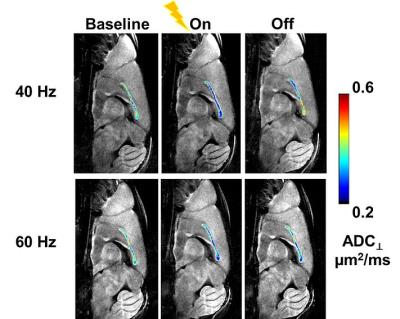
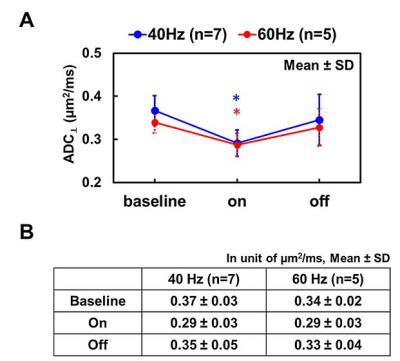
Figure 3 Compared to its baseline, ADC⊥ decreased during electrical stimulation and returned towards baseline level post-stimulation (A and B). Group results revealed a 21% and 15% stimulation-associated ADC⊥ decrease for 40 and 60 Hz stimulation.
* indicates p < 0.05, comparing to its baseline