0447
RACE-GRASP: Respiratory-weighted and Aortic Contrast Enhancement-guided GRASP MRI1Center for Advanced Imaging Innovation and Research (CAI2R), New York University School of Medicine, New York, NY, United States
Synopsis
This work proposes a technique named RACE-GRASP (Respiratory-weighted and Aortic Contrast Enhancement guided Golden-angle RAdial Sparse Parallel imaging) for robust free-breathing DCE-MRI of the liver. First, the aortic contrast enhancement curve is automatically detected from continuously acquired data and is used to guide k-space sorting to ensure precise capture of desired contrast-enhancement phases. Second, k-space data of each contrast phase are binned into different respiratory motion states, and each motion bin is weighted differently during image reconstruction to reduce motion blurring. The performance of RACE-GRASP was demonstrated in 5 healthy volunteers and was compared with conventional GRASP.
INTRODUCTION
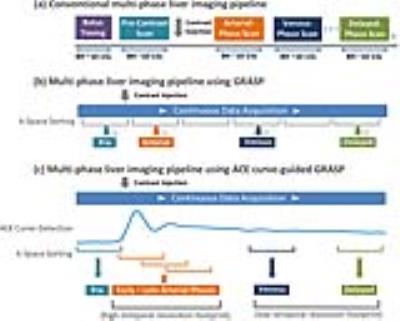
In routine DCE-liver imaging, separate 3D images at desired contrast-enhanced phases are acquired during multiple breath-holds. In order to ensure precise capture of contrast-enhancement phases, a bolus timing step is always performed, in which the aortic contrast enhancement (ACE) curve is obtained from a manually placed ROI in the aorta. This punctuated imaging pipeline (Figure1a) is cumbersome and inefficient for routine liver MR exams. GRASP (Golden-angle RAdial Sparse Parallel) MRI1 is a promising technique to overcome some of these limitations. It enables continuous data acquisitions using golden-angle radial sampling and can reconstruct dynamic images with different temporal resolutions combining compressed sensing and parallel imaging (Figure1b). However, the current GRASP framework suffers from a few limitations. First, since the timing step is skipped, optimal capture of desired phases (e.g., arterial phases) is not guaranteed from a default reconstructed scheme. Second, radiologists must manually select desired phases from a series of contrast-enhancement images, which is time consuming. Third, despite the improved motion robustness of radial sampling, respiratory motion blurring is often observed in GRASP, particularly for patients with irregular and deep breathing. Here, we propose a new technique called RACE-GRASP (Respiratory-weighted and Aortic Contrast Enhancement-guided GRASP), which performs automatic ACE curve-guided k-space sorting and respiratory-weighted reconstruction to overcome these challenges.METHODS
ACE Curve-Guided Data Sorting: Figure1c shows the proposed k-space sorting scheme, in which data are sorted with a high-temporal-resolution footprint for arterial phases (fast contrast passage period) and low-temporal-resolution footprint for venous and delayed phases (slow contrast wash-out period). More importantly, the ACE curve ensures optimal capture of desired phases, with a role similar to conventional bolus timing. Figure2 summarizes the pipeline for ACE curve detection. First, a low spatial resolution but high temporal resolution 3D image series is reconstructed, from which a temporal signal evolution curve is calculated for a 6x6 kernel that transverses the entire FOV for each slice. Since the aorta is presented in most slices, and its contrast enhancement has a higher slope than most other regions in the body, the curve with the steepest variation would be the ACE curve in most slices (Figure2a). In order to exclude false detection (e.g., regions close to the heart), a clustering method proposed in (2) is applied. Specifically, a correlation matrix of all curves (one from each slice) is calculated, followed by a thresholding process to generate a binary mask (Figure2b) for identifying the dominant curve pattern (ACE curve in our scenario, Figure2c).
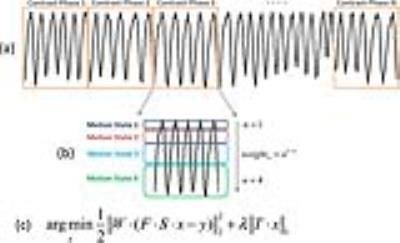
Respiratory-Weighted Reconstruction: As shown in Figure3a&b, each contrast phase is binned into four motion states spanning from end-expiration to end-inspiration, using a respiratory motion signal extracted from the acquired data3. Each motion state is then weighted differently in image reconstruction in order to reduce the contribution of motion-corrupted k-space measurements to the final images4,5. Figure3c shows the cost function for image reconstruction, in which the weight W decays exponentially from motion state 1 (expiration) to motion state 4 (inspiration)5.
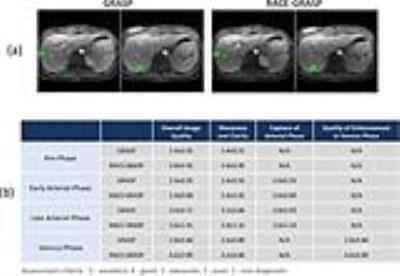
Imaging Study: IRB-approved golden-angle radial liver MRI was performed in 5 volunteers using a DCE-imaging protocol described in (1). GRASP images were reconstructed with a ~15s temporal resolution. For RACE-GRASP, each contrast phase was reconstructed with a temporal footprint of ~15s and was reconstructed at the temporal positions of (-20, -10, 0, +5, +10, +15, +22, +30, +45, +60, +90, +120) seconds. Here, 0s indicates the peak aortic enhancement position and -/+ indicates whether the current phase is before or after the peak aortic enhancement. The phases at +5s, +10s and +15s were exported as the early-late arterial phases and the phase at +60s was exported as the venous phase. One radiologist manually selected the best pre-contrast, early-arterial, late-arterial and venous phases for GRASP, and they were compared to the corresponding phases in RACE-GRASP. Another radiologist blinded to this study scored the overall image quality, vessel sharpness/clarity and the capture of the arterial phase based on a 5-point scale (5 to 1: excellent to non-diagnostic).
RESULTS
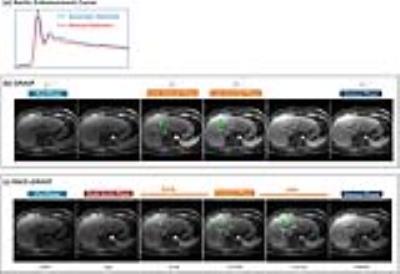
Figure4a compares representative ACE curves detected automatically as opposed to manually. The curves correlated well with each other. RACE-GRASP enabled better capture of the arterial phases comparing to GRASP (green arrows, Figure4b&c). Figure5a compares a representative venous phase from GRASP and RACE-GRASP. RACE-GRASP achieved clearly improved vessel clarity/sharpness and vessel-tissue contrast (green arrows). The image quality improvement is confirmed by the scores in Figure5b.DISCUSSION
RACE-GRASP represents a more robust framework for rapid and continuous DCE-MRI. It enables precise capture of desired contrast-enhanced phases without a separate bolus timing step. Meanwhile, it achieves superior image quality to conventional GRASP without increasing reconstruction time.Acknowledgements
This work was supported in part by the NIH, and was performed under the rubric of the Center for Advanced Imaging Innovation and Research (CAI2R), a NIBIB Biomedical Technology Resource Center (NIH P41 EB017183).References
[1] Feng L et al. MRM 2014 72 (3), 707-717 [2] Zhang T et al. MRM 2016 76:197–205 [3] Feng L et al. MRM 2016 75 (2), 775-788 [4] Johnson KM et al. MRM 2012 67:1600-1608 [5] Zhang T et al. JMRI 2015 41:460–473Figures