0442
Wave-CAIPI for Highly Accelerated MP-RAGE Imaging1Massachusetts General Hospital, Boston, MA, United States, 2German Cancer Resarch Center, Heidelberg, Germany, 3Harvard Medical School, Boston, MA, United States, 4Harvard-MIT Health Sciences and Technology, Boston, MA, United States, 5Boston Children’s Hospital, Boston, MA, United States, 6Siemens Medical Solutions Inc, Malvern, PA, United States
Synopsis
We introduce a highly accelerated T1-weighted MP-RAGE acquisition that utilizes a novel reordering scheme and Wave-CAIPI encoding to retain high image quality. R=9-fold accelerated in vivo MP-RAGE scans were performed in 71 sec, with maximum and average g-factor of gmax=1.27 and gavg=1.06. Compared to the state-of-the-art 2D-CAIPIRINHA method, this is a factor of 4.6/1.4 improvement in gmax/gavg. In addition, we demonstrate a 57 sec acquisition at 7T with R=12-fold acceleration. This acquisition had a g-factor performance of gmax=1.15 and gavg=1.04. Wave encoding overcomes the g-factor noise amplification penalty and allows for an order of magnitude acceleration of MP-RAGE acquisitions.
Introduction
MP-RAGE1 is widely used in neuroimaging and for clinical applications as it provides detailed contrast between gray matter, white matter and cerebrospinal fluid. To achieve T1w contrast, MP-RAGE utilizes a magnetization preparation, gradient echo readout and recovery period. This corresponds to long scan times which may increase susceptibility to patient motion and reduce patient comfort. While established parallel imaging methods like SENSE2 and GRAPPA3 allow up to 3-fold reduction in scan time, we propose Wave-CAIPI MP-RAGE, which enables more than an order of magnitude acceleration and below 1 min scan time.Theory
Wave-CAIPI4 extends the conventional gradient echo readout by sinusoidal waveforms on the Gy and Gz gradients during the sampling period, which produces voxel spreading along the x (readout) axis. As the amount of readout spreading is dependent on the y and z spatial positions, Wave encoding improves the coil sensitivity variation in the collapsed voxels for accelerated acquisitions.Methods
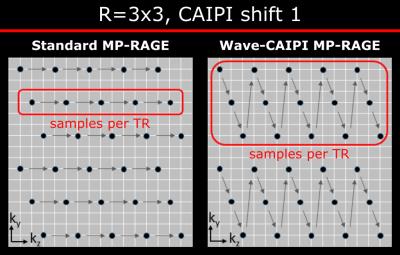
In conventional MP-RAGE acceleration along the partition encoding only shortens the echo train length of the gradient echo readout without affecting the acquisition time5. For this reason, Wave-CAIPI MP-RAGE utilizes a novel scheme (Fig. 1) that merges Rz planes of kx-kz k-space in an interleaved fashion. This ensures that the k-space center of each plane is acquired close to the inversion time (TI) preserving the T1w contrast.
In compliance with IRB requirements a healthy volunteer was scanned on a 3T Siemens Skyra scanner using a 32-channel product coil. We achieved 69 sec scan time at R=3x3 acceleration with TE/TR/TI = 3.8/2500/1100 ms, CAIPI shift 1 and 11 sinusoidal cycles per readout at 8.8 mTm-1 maximum gradient amplitude. In addition, a 1.8 s calibration scan (GRE) was acquired to compute the coil sensitivity profile using ESPIRiT6. A conventional 2D-CAIPI7 scan without wave gradients served as a benchmark for comparison. The reconstruction of all presented datasets and corresponding g-factor maps were performed in MATLAB. To assess the quality of Wave-CAIPI in comparison to techniques routinely used in clinical settings, we acquired three averages of Wave-CAIPI using the protocol described above (total scan time 3 min 23 sec) and a R=4x1 GRAPPA measurement of similar duration (3 min 14 sec including 24 integrated ACS lines). Even higher acceleration for a 1mm isotropic full brain scan was achieved on a Siemens Magnetom 7T scanner. At R=4x3 acceleration the measurement time was reduced to 57 sec. Due to physiological constraints 9 sinusoidal cycles and 16.0 mTm-1 maximum gradient amplitude were chosen. We also performed a series of g-factor simulations for various number of sinusoidal cycles and maximum gradient slew rates to investigate the wave parameter space. Furthermore, the effect of T1 recovery and small flip angle excitation in Wave-CAIPI MP-RAGE (echo spacing 8 ms, flip angle 9°, 10 mTm-1 maximum gradient amplitude) was simulated for a homogenous object of T1=1500ms.
Results
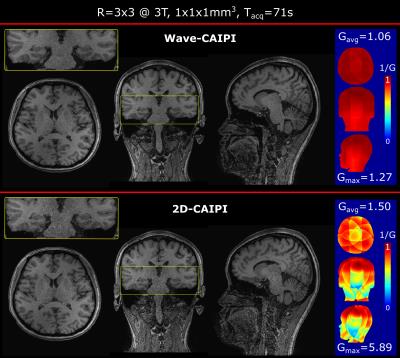
Figure 2 shows Wave-CAIPI and 2D-CAIPI acquisitions at R=3x3 acceleration with corresponding g-factor maps. While 2D-CAIPI suffered from severe noise amplification particularly in the brain stem, Wave-CAIPI demonstrated much enhanced encoding performance and 1.5/4.6-fold improvement in avg./max. g-factor.
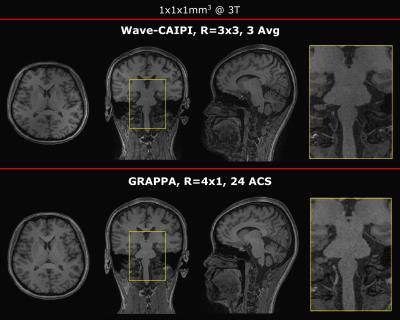
Furthermore, a time matched R=4x1 GRAPPA scan was compared to three averages of R=3x3 Wave-CAIPI MP-RAGE (Fig. 3). Both acquisitions provided comparable image quality, SNR and T1-weighted contrast. However, Wave-CAIPI is anticipated to provide increased robustness to patient motion, as motion-corrupted averages can be discarded.
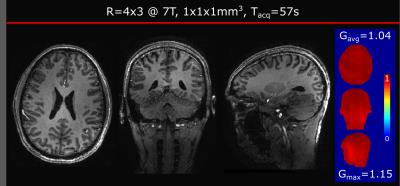
More than an order of magnitude acceleration (R=12) was achieved at 7T with corresponding scan time of 57 sec. Despite the high loss of SNR due to the intrinsic sqrt(R) penalty, all three views of Fig. 4 show detailed contrast and image quality.
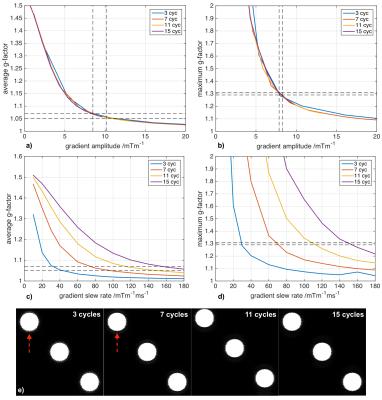
Figure 5 depicts the results of the wave parameter investigation. It revealed that the g-factor is mainly determined by the maximum gradient amplitude and mostly independent of the number of cycles. However, increased number of cycles was found to reduce artefacts arising from T1 recovery (Fig. 5e).
Discussion and conclusion
Wave-CAIPI MP-RAGE overcomes the g-factor noise amplification penalty for highly accelerated imaging and allows more than an order of magnitude acceleration corresponding to less than one minute scan time. This fast sequence is expected to benefit clinical and research applications that routinely employ MP-RAGE for T1 weighted imaging.Acknowledgements
Grant Support: NIH R24MH106096, R01EB020613, R01EB017337, U01HD087211, R01EB019437, R24MH106053, P41EB015896References
[1] J. P. Mugler, J. R. Brookeman. Three-dimensional magnetization-prepared rapid gradient-echo imaging (3D MP RAGE). Magn. Reson. Med. 1990;15(1):152–157.
[2] K. P. Pruessmann, M. Weiger, M. B. Scheidegger, P. Boesiger. SENSE: Sensitivity encoding for fast MRI. Magn. Reson. Med. 1999;42(5):952–962.
[3] M. A. Griswold, P. M. Jakob, R. M. Heidemann, M. Nittka, V. Jellus, J. Wang, B. Kiefer, A. Haase. Generalized autocalibrating partially parallel acquisitions (GRAPPA). 2002;47(6):1202–1210.
[4] B. Bilgic, B. A. Gagoski, S. F. Cauley, A. P. Fan, J. R. Polimeni, P. E. Grant, L. L. Wald, and K. Setsompop. Wave-CAIPI for highly accelerated 3D imaging. Magn. Reson. Med. 2015;73(6):2152–2162.
[5] K. Setsompop, D. A. Feinberg, J. R. Polimeni. Rapid brain MRI acquisition techniques at ultra-high fields. NMR Biomed. 2016;29:1198-1221.
[6] M. Uecker, P. Lai, M. J. Murphy, P. Virtue, M. Elad, J. M. Pauly, S. S. Vasanawala, M. Lustig, ESPIRiT--an eigenvalue approach to autocalibrating parallel MRI: where SENSE meets GRAPPA. Magn. Reson. Med. 2014;71(3):990–1001.
[7] F. A. Breuer, M. Blaimer, M. F. Mueller, N. Seiberlich, R. M. Heidemann, M. A. Griswold, P. M. Jakob. Controlled Aliasing in Volumetric Parallel Imaging (2D CAIPIRINHA). 2006;55(3):549–556.
Figures