0438
Prognostic Role of Liver Stiffness Measurement Using Magnetic Resonance Elastography in Patients with Compensated Chronic Liver Disease1Radiology, Seoul National University Hospital, Seoul, Korea, Republic of, 2Korea, Republic of
Synopsis
Liver stiffness measurement (LSM) using magnetic resonance elastography (MRE) can estimate the degree of liver fibrosis. We retrospectively evaluate the prognostic role of LSM using MRE in compensated chronic liver disease patients. A total of 217 patients with compensated chronic liver disease who underwent MRE were included. After a mean and median follow-up of 44.5±17.8 months and 46.0 months, LSM value obtained from MRE was turned out to be significant predictive factor for overall survival, development of hepatic decompensation and occurrence of hepatocellular carcinoma.
Background & Aims: Liver stiffness measurement (LSM) using magnetic resonance elastography (MRE) can estimate the degree of liver fibrosis. We retrospectively evaluate the prognostic role of LSM using MRE in compensated chronic liver disease patients.
Methods: We enrolled a total of 217 patients with compensated chronic liver disease who underwent MRE between January 2010 and December 2013. LSM value was obtained from MRE in each patients. After a mean and median follow-up of 44.5 ± 17.8 months and 46.0 months, respectively, cumulative incidence (CI) of hepatocellular carcinoma (HCC) occurrence and development of decompensation as well as overall survival (OS) were estimated using Kaplan-Meier method and its prognostic factors were evaluated using Cox proportional hazard regression model.
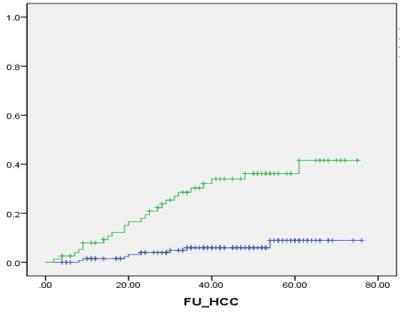
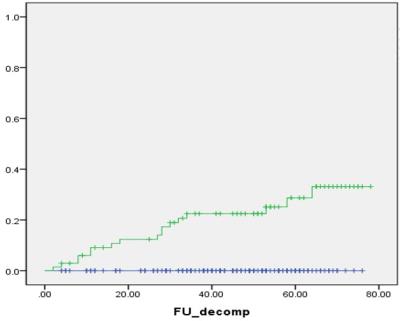
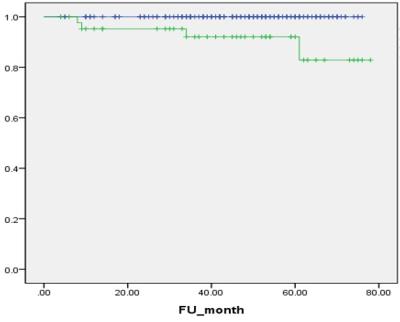
Results: During the follow-up period, HCC occurred in 33 patients, and 1-, 3- and 5-year CI of HCC occurrence were 3.8%, 14.8% and 18.9%, respectively. LSM value was a significant predictive factor for HCC occurrence (P<0.001, Hazard ratio [HR] = 1.47 per unit [1.21-1.79]). Seventeen patients were experienced hepatic decompensation, and 1-, 3- and 5-year CI of decompensation were 2.8%, 7.3% and 10.3%, respectively. LSM value was also significantly associated with development of decompensation (P<0.001, HR=1.87 per unit [1.37-2.54]). Four patients died, and 1-, 3- and 5-year OS were 99.1%, 98.4% and 96.2%, respectively. LSM value was the only significant affecting factor for OS (P<0.001, HR=1.98 per unit [1.36-2.86]).
Conclusions: LSM values obtained from MRE was a significant predictive factor for development of decompensation, HCC occurrence as well as OS in compensated chronic liver disease patients.
Background & Aims: Liver stiffness measurement (LSM) using magnetic resonance elastography (MRE) can estimate the degree of liver fibrosis. We retrospectively evaluate the prognostic role of LSM using MRE in compensated chronic liver disease patients.
Methods: We enrolled a total of 217 patients with compensated chronic liver disease who underwent MRE between January 2010 and December 2013. LSM value was obtained from MRE in each patients. After a mean and median follow-up of 44.5 ± 17.8 months and 46.0 months, respectively, cumulative incidence (CI) of hepatocellular carcinoma (HCC) occurrence and development of decompensation as well as overall survival (OS) were estimated using Kaplan-Meier method and its prognostic factors were evaluated using Cox proportional hazard regression model.
Results: During the follow-up period, HCC occurred in 33 patients, and 1-, 3- and 5-year CI of HCC occurrence were 3.8%, 14.8% and 18.9%, respectively. LSM value was a significant predictive factor for HCC occurrence (P<0.001, Hazard ratio [HR] = 1.47 per unit [1.21-1.79]). Seventeen patients were experienced hepatic decompensation, and 1-, 3- and 5-year CI of decompensation were 2.8%, 7.3% and 10.3%, respectively. LSM value was also significantly associated with development of decompensation (P<0.001, HR=1.87 per unit [1.37-2.54]). Four patients died, and 1-, 3- and 5-year OS were 99.1%, 98.4% and 96.2%, respectively. LSM value was the only significant affecting factor for OS (P<0.001, HR=1.98 per unit [1.36-2.86]).
Conclusions: LSM values obtained from MRE was a significant predictive factor for development of decompensation, HCC occurrence as well as OS in compensated chronic liver disease patients.
Acknowledgements
No acknowledgement found.References
1. Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2095-128. 2. Blachier M, Leleu H, Peck-Radosavljevic M, et al. The burden of liver disease in Europe: a review of available epidemiological data. J Hepatol 2013;58:593-608. 3. Chang W, Lee JM, Yoon JH, et al. Liver Fibrosis Staging with MR Elastography: Comparison of Diagnostic Performance between Patients with Chronic Hepatitis B and Those with Other Etiologic Causes. Radiology 2016;280:88-97. 4. Manos MM, Leyden WA, Murphy RC, et al. Limitations of conventionally derived chronic liver disease mortality rates: Results of a comprehensive assessment. Hepatology 2008;47:1150-7. 5. Asrani SK, Larson JJ, Yawn B, et al. Underestimation of liver-related mortality in the United States. Gastroenterology 2013;145:375-82 e1-2. 6. Garcia-Tsao G, Friedman S, Iredale J, et al. Now there are many (stages) where before there was one: In search of a pathophysiological classification of cirrhosis. Hepatology 2010;51:1445-9. 7. D'Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol 2006;44:217-31. 8. Schuppan D, Afdhal NH. Liver cirrhosis. Lancet 2008;371:838-51. 9. Fleming KM, Aithal GP, Card TR, et al. The rate of decompensation and clinical progression of disease in people with cirrhosis: a cohort study. Aliment Pharmacol Ther 2010;32:1343-50. 10. Parkin DM, Bray F, Ferlay J, et al. Estimating the world cancer burden: Globocan 2000. Int J Cancer 2001;94:153-6. 11. Lee DH, Lee JM, Lee JY, et al. Radiofrequency ablation of hepatocellular carcinoma as first-line treatment: long-term results and prognostic factors in 162 patients with cirrhosis. Radiology 2014;270:900-9. 12. Singh S, Fujii LL, Murad MH, et al. Liver stiffness is associated with risk of decompensation, liver cancer, and death in patients with chronic liver diseases: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2013;11:1573-84 e1-2; quiz e88-9. 13. Castera L, Vergniol J, Foucher J, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology 2005;128:343-50. 14. Foucher J, Chanteloup E, Vergniol J, et al. Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study. Gut 2006;55:403-8. 15. Cassinotto C, Lapuyade B, Mouries A, et al. Non-invasive assessment of liver fibrosis with impulse elastography: comparison of Supersonic Shear Imaging with ARFI and FibroScan(R). J Hepatol 2014;61:550-7. 16. Robic MA, Procopet B, Metivier S, et al. Liver stiffness accurately predicts portal hypertension related complications in patients with chronic liver disease: a prospective study. J Hepatol 2011;55:1017-24. 17. Corpechot C, Carrat F, Poujol-Robert A, et al. Noninvasive elastography-based assessment of liver fibrosis progression and prognosis in primary biliary cirrhosis. Hepatology 2012;56:198-208. 18. Jung KS, Kim SU, Ahn SH, et al. Risk assessment of hepatitis B virus-related hepatocellular carcinoma development using liver stiffness measurement (FibroScan). Hepatology 2011;53:885-94. 19. Wong GL, Chan HL, Wong CK, et al. Liver stiffness-based optimization of hepatocellular carcinoma risk score in patients with chronic hepatitis B. J Hepatol 2014;60:339-45. 20. Kim MN, Kim SU, Kim BK, et al. Increased risk of hepatocellular carcinoma in chronic hepatitis B patients with transient elastography-defined subclinical cirrhosis. Hepatology 2015;61:1851-9. 21. Huwart L, Sempoux C, Salameh N, et al. Liver fibrosis: noninvasive assessment with MR elastography versus aspartate aminotransferase-to-platelet ratio index. Radiology 2007;245:458-66. 22. Loomba R, Wolfson T, Ang B, et al. Magnetic resonance elastography predicts advanced fibrosis in patients with nonalcoholic fatty liver disease: a prospective study. Hepatology 2014;60:1920-8. 23. Huwart L, Sempoux C, Vicaut E, et al. Magnetic resonance elastography for the noninvasive staging of liver fibrosis. Gastroenterology 2008;135:32-40. 24. Van Beers BE, Daire JL, Garteiser P. New imaging techniques for liver diseases. J Hepatol 2015;62:690-700. 25. Lee DH, Lee JM, Yi NJ, et al. Hepatic stiffness measurement by using MR elastography: prognostic values after hepatic resection for hepatocellular carcinoma. Eur Radiol 2016. 26. Asrani SK, Talwalkar JA, Kamath PS, et al. Role of magnetic resonance elastography in compensated and decompensated liver disease. J Hepatol 2014;60:934-9. 27. Lee DH, Lee JM, Han JK, et al. MR elastography of healthy liver parenchyma: Normal value and reliability of the liver stiffness value measurement. J Magn Reson Imaging 2013;38:1215-23. 28. Wang Y, Ganger DR, Levitsky J, et al. Assessment of chronic hepatitis and fibrosis: comparison of MR elastography and diffusion-weighted imaging. AJR Am J Roentgenol 2011;196:553-61. 29. Shin SU, Lee JM, Yu MH, et al. Prediction of esophageal varices in patients with cirrhosis: usefulness of three-dimensional MR elastography with echo-planar imaging technique. Radiology 2014;272:143-53. 30. European Association For The Study Of The L, European Organisation For R, Treatment Of C. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908-43. 31. Faraggi D, Simon R. A simulation study of cross-validation for selecting an optimal cutpoint in univariate survival analysis. Stat Med 1996;15:2203-13. 32. Minagawa M, Ikai I, Matsuyama Y, et al. Staging of hepatocellular carcinoma: assessment of the Japanese TNM and AJCC/UICC TNM systems in a cohort of 13,772 patients in Japan. Ann Surg 2007;245:909-22. 33. Llop E, Berzigotti A, Reig M, et al. Assessment of portal hypertension by transient elastography in patients with compensated cirrhosis and potentially resectable liver tumors. J Hepatol 2012;56:103-8. 34. Choi SY, Jeong WK, Kim Y, et al. Shear-wave elastography: a noninvasive tool for monitoring changing hepatic venous pressure gradients in patients with cirrhosis. Radiology 2014;273:917-26. 35. Castera L, Pinzani M, Bosch J. Non invasive evaluation of portal hypertension using transient elastography. J Hepatol 2012;56:696-703. 36. Shi KQ, Fan YC, Pan ZZ, et al. Transient elastography: a meta-analysis of diagnostic accuracy in evaluation of portal hypertension in chronic liver disease. Liver Int 2013;33:62-71. 37. Vergniol J, Foucher J, Terrebonne E, et al. Noninvasive tests for fibrosis and liver stiffness predict 5-year outcomes of patients with chronic hepatitis C. Gastroenterology 2011;140:1970-9, 1979 e1-3. 38. Lee HW, Yoo EJ, Kim BK, et al. Prediction of development of liver-related events by transient elastography in hepatitis B patients with complete virological response on antiviral therapy. Am J Gastroenterol 2014;109:1241-9. 39. Pang JX, Zimmer S, Niu S, et al. Liver stiffness by transient elastography predicts liver-related complications and mortality in patients with chronic liver disease. PLoS One 2014;9:e95776. 40. Kim SY, An J, Lim YS, et al. MRI With Liver-Specific Contrast for Surveillance of Patients With Cirrhosis at High Risk of Hepatocellular Carcinoma. JAMA Oncol 2016.Figures