0433
Quantitative Free-Breathing Dynamic Contrast-enhanced MRI in Hepatocellular Carcinoma Using Gd-EOB-DTPA:Correlations With Ki67 Proliferation Status and Histological Grades1West China Medical School of Sichuan University, Chengdu, People's Republic of China, 2Departmento of Radiology, West China Hospital of Sichuan University, Chengdu, People's Republic of China
Synopsis
This study aims to validate a free-breathing DCE-MRI in HCC patients using gadoxetic acid, and to determine the relationship between DCE-MRI parameters and histological results. Perfusion parameters (Ktrans, Kep, Ve and iAUC) from 34 patients were compared with CT results and correlated with Ki67 indices and the histological grades of HCC. Significant relationship was found between DCE-MRI and CT results, indicating the validity of this protocol in evaluating the vascular change of HCC. The DCE-MRI derived Ktrans and iAUC were significantly correlated with the histological grades of HCC, suggesting the potential of those parameters in predicting the malignancy of tumors.
Purpose
Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) can provide quantitative and biological relevant information related to tumor malignancy and angiogenesis. Although DCE-MRI has been frequently applied, the relationship between those perfusion parameters and histopathology has not yet been fully understood. The purpose of this study was to validate a free-breathing DCE-MRI in hepatocellular carcinoma (HCC) patients using gadoxetic acid, by comparing it with conventional contrast-enhanced computed tomography (CT), and to explore its clinical significance by correlating the DCE-MRI derived quantitative parameters with histological results.Methods
Thirty-four HCC patients were included in this prospective study. Patients were examined preoperatively using a 3.0 Tesla scanner (Magnetom Skyra, Siemens Healthcare, Erlangen, Germany). The free-breathing DCE-MRI data was acquired using a prototype radial stack-of-stars three-dimensional (3D) spoiled gradient echo pulse sequence with golden-angle radial sampling schemes over the course of 6:15 minutes. Post-processing of DCE MRI data was performed on the Tissue4D software (Siemens Healthcare) to calculate the Ktrans, Kep, Ve and iAUC using a single-input two-compartment model based on the Toft’s model. The Ki-67 labeling index and Edmondson-Steiner classification of HCC were histopathologically determined.
Perfusion parameters of HCC were compared with CT results. Differences of DCE-MRI parameters between tumors and background liver parenchyma (BLP) were compared using paired t-tests. The DCE-MRI derived Ktrans, Kep, Ve and iAUC were correlated with Ki67 indices and histological grades of HCC by Spearman’s (or Pearson’s) correlation analysis. Differences of perfusion parameters between different histopathological groups were compared. Receiver operation characteristic (ROC) analysis of discriminating low-grades (grade 1 and 2) from high-grades (grade 3 and 4) HCC was performed for perfusion parameters.
Results
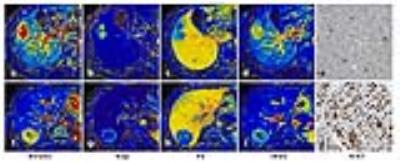
The Ktrans and iAUC were both significantly correlated with the degree of arterial enhancement (AE) and portal venous enhancement (PVE) of HCC at CT (rho=0.549, P=0.002 for Ktrans vs. AE, and rho=0.447, P=0.015 for Ktrans vs. PVE; r=0.546, P=0.002 for iAUC vs. AE, and r=0.558, P=0.002 for iAUC vs. PVE). Figure 1 shows the scatterplots of median values of AE versus median values of Ktrans and iAUC.
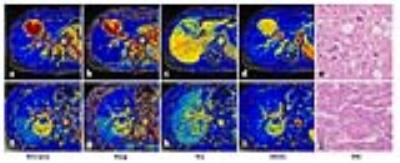
The Ktrans, Kep and iAUC of HCC were significantly higher than that of BLP (P<0.001 for all), and the Ve of HCC was significantly lower than that of BLP (P<0.001). A significant relationship between Ktrans and the Ki67 index of HCC was observed (rho=-0.408, P=0.017) (Figure 2). The Ktrans and iAUC were significantly correlated with the Edmondson-Steiner grade of HCC (rho=-0.444, P=0.009 for Ktrans, and rho=-0.523, P=0.002 for iAUC), while no significant correlation with the histological grade was observed for Kep and Ve (P>0.05 for all). The Ktrans, Kep and iAUC of high grades HCC were significantly lower than that of low grades HCC (P=0.001, 0.031 and 0.003 respectively), but there was no statistically significant differences for Ve between the two groups (P>0.05)(Figure 3). Ktrans, Kep and iAUC demonstrated moderate diagnostic performance (iAUC=0.78, 0.77 and 0.80, respectively) for discriminating low-grades from high-grades HCC without significant differences.
Discussion
With the radial acquisition with golden-angle scheme, we performed a free-breathing quantitative DCE-MRI for liver imaging. Vascular changes in HCC can be reliably detected and quantified by this method without distinct motion artifact. The relationships between DCE-MRI and CT results could suggest the validity of this method in evaluating the vascular changes of HCC. The increased Ktrans was considered to reflect an increased vascular permeability in HCC, and the decreased Ve, which was mainly determined by the interstitial distribution space, indicated an aggressive growth of tumor cells.1,2
Of the various perfusion parameters, Ktrans is the most commonly used DCE-MRI parameters. It reflects a combination of blood flow, vessel permeability and vessel density.3 On the other hand, the semi-quantitative parameter, iAUC, is associated with tumor blood influx, perfusion and interstitium, representing the general tumor blood flow, overall perfusion and tumor interstitial space index.3 It is a relatively robust and simple kinetic parameter to derive and is able to characterize all enhancing regions without problems associated with model fitting failures.4 Therefore, Ktrans and iAUC were expected to better represent the actual biological change of HCC than other parameters. The results that both Ktrans and iAUC were correlated significantly with the histological grade of HCC in the present study suggested the potential of those parameters in predicting the malignancy of tumors.
Conclusion
This free-breathing quantitative DCE-MRI protocol provides a valid and practicable method for future liver perfusion imaging. The DCE-MRI derived Ktrans and iAUC were significantly correlated with the histological grade of HCC.Acknowledgements
No acknowledgement found.References
1. Chen BB, Shih TT. DCE-MRI in hepatocellular carcinoma-clinical and therapeutic image biomarker. World journal of gastroenterology 2014;20(12):3125-3134.
2. Zwick S, Brix G, Tofts PS, et al. Simulation-based comparison of two approaches frequently used for dynamic contrast-enhanced MRI. European radiology 2010;20(2):432-442.
3. Tofts PS, Brix G, Buckley DL, et al. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. Journal of magnetic resonance imaging : JMRI 1999;10(3):223-232.
4. Padhani AR, Khan AA. Diffusion-weighted (DW) and dynamic contrast-enhanced (DCE) magnetic resonance imaging (MRI) for monitoring anticancer therapy. Targeted oncology 2010;5(1):39-52.
Figures