0431
Longitudinal Characterization of Liver Regeneration and Portal Hemodynamics in Living Donor Liver Transplant1Radiology, University of Wisconsin, Madison, WI, United States, 2Mechanical Engineering, University of Wisconsin, Madison, WI, United States, 3Biomedical Engineering, University of Wisconsin, Madison, WI, United States, 4Surgery, University of Wisconsin, Madison, WI, United States, 5Medical Physics, University of Wisconsin, Madison, United States, 6Medicine, University of Wisconsin, Madison, United States
Synopsis
The purpose of this study was to evaluate longitudinal liver regeneration and hemodynamic changes of living donor liver transplant (LDLT) donors in response to surgical liver resection. Five living related liver donors were studied. Subjects were imaged using 4D Flow MRI before and at several times following partial hepatectomy. The ability to longitudinally evaluate liver regeneration and portal hemodynamic changes non-invasively demonstrates that 4D flow MRI is a suitable tool for both surgical planning of LDLT, and for improved understanding of the liver regeneration and hemodynamic changes that occur in the remnant liver of the donor.
Introduction:
More than 36,000 people die annually from cirrhosis and end-stage liver disease (ESLD) in the US, and 20,000 from hepatocellular carcinoma (HCC)1, a common consequence of cirrhosis. Liver transplantation is a highly successful, definitive therapy for patients with ESLD. However, in the past two decades, the growing disparity between the number of liver transplant candidates and the supply of deceased donor organs has increased the need for living donor liver transplantation (LDLT) as an alternative, which has shown success rates comparable to those in cadaveric liver transplants. Partial hepatectomy in live donors is a major surgical intervention that carries small but real risks for the donor2. To minimize unintended complications, a comprehensive, non-invasive assessment of donors beyond the current standard of care is of paramount importance to increase living donor safety. Therefore, the purpose of this study was to evaluate longitudinal liver regeneration and hemodynamic changes of LDLT donors in response to surgical liver resection.Methods:
Human Subjects: In this IRB-approved and HIPAA-compliant study, 5 LDLT donors (2M, 3F; 30-53 years; 58-86 Kg) were imaged after written informed consent before and at different time points after surgery (4 patients right lobe and 1patient left lateral lobe resection). Subjects were scanned after >5 hours of fasting (pre-surgery) to avoid variability in splanchnic flow after a meal3.
MR-Imaging: Studies were conducted on a clinical 3T MRI system (MR750, GE Healthcare, Waukesha, WI) using a 32-channel phased array torso coil (NeoCoil, Pewaukee, WI). 4D velocity mapping was achieved using a cardiac-gated time-resolved 3D radially undersampled phase contrast acquisition (5-point PC-VIPR) with increased velocity sensitivity performance4,5. Radial 4D flow MRI image parameters included: imaging volume: 32x32x24cm spherical, 1.25mm acquired isotropic spatial resolution, TR/TE=6.4/2.2ms. All subjects received 0.05 mmol/kg of gadoxetic acid (Eovist, Bayer Healthcare, Wayne, NJ)) for the pre-surgery scan only, as part of their clinical evaluation for other purposes. Post-surgery 4D flow MRI imaging protocols were identical but no contrast was used.
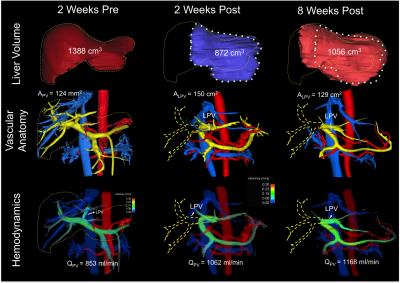
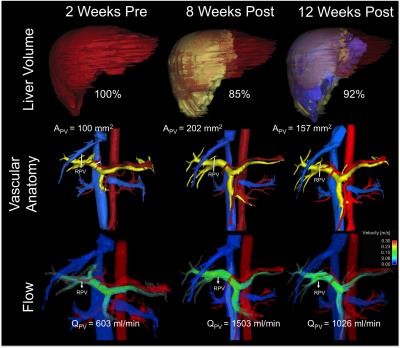
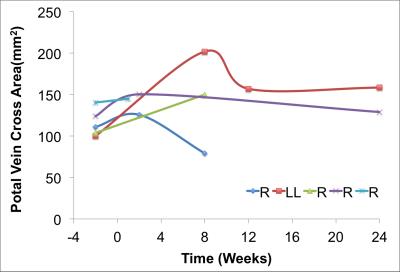
4D flow MRI Data Analysis: Liver volume quantification and vessel segmentation were performed in MIMICs (Materialize, Leuven, Belgium) from the 4D flow magnitude images and PC angiograms respectively. Manual placement of cut-planes in the vessel of interest where flow measurements and visualizations were conducted took place in EnSight (CEI, Apex, NC). Flow, peak velocity and cross sectional area of the vessel were quantified at the left (LPV), right (RPV) and main portal Vein (PV) (Figures 1 and 2). Remnant liver volume (RLV) day of donation was determined by subtracting the estimated resected lobe graft volume from the total liver volume (TLV) measured from MRI before surgery. Statistics: Liver volume, blood flow and cross-sectional area of the portal veins were compared before and after the surgery. Given the variability in post-surgery evaluation times results are presented as absolute patient specific values.
Results and Discussion:
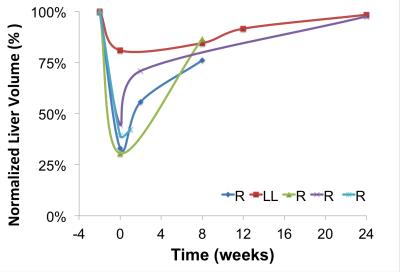
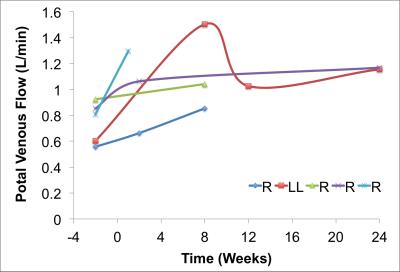
Segmentation of 4D Flow MRI magnitude images was successful and provided accurate information about liver volumes. Similarly, PC angiograms were successfully segmented for vessel area quantification. In average the four right lobe donors kept a 36 ± 6 % of the TLV, whereas the left lateral lobe donor kept 80% of the TLV as remnant liver volume. Figure 3 shows the time progression of the liver growth after partial hepatectomy (regeneration). 3 of the 4 right lobe donors were scanned within two weeks after surgery showing a rapid regeneration (45 ± 34 % of RLV). After 8 weeks the liver volume in the right lobe donors was 82 ± 5 % of TLV. Finally, two (1 right, 1 left) subjects scanned six months (24 weeks) after surgery showed near complete regeneration (98% of TLV). Portal blood flow increased immediately after surgery and returned to normal values as liver regenerated in time (Figure 4). Similar trend was observed for the cross-sectional area (Fig.5)Summary:
Dramatic anatomical and hemodynamic changes in the liver result after partial hepatectomy. With more than 60% of the liver resected, the remaining liver must accommodate increased flow from the portomesenteric circulation with an increased vascular resistance (R) due to the limited central vascularity (e.g., changes in left portal vein after right hepatectomy). Changes in portal venous flow, pressure and wall shear stress have been suggested as the primary stimulus/impediment in triggering liver regeneration after partial hepatectomy (ref). The ability to quantify liver regeneration (volume) and hemodynamic changes in the portal circulation longitudinally and non-invasively demonstrates that 4D flow MRI may be a suitable tool for both surgical planning of LDLT. Further, 4D flow MRI may contribute to improved understanding of the hemodynamic changes that occur in the remnant liver of the donor such as the patient specific polymorphism in liver regeneration (Fig1).Acknowledgements
We acknowledge support from the NIH (R01 DK088925) the AHA (14SDG19690010), UW Radiology R&D and GE HealthcareReferences
1. Mokdad AA, Lopez AD, Shahraz S, Lozano R, Mokdad AH, Stanaway J, et al. Liver cirrhosis mortality in 187 countries between 1980 and 2010: a systematic analysis. BMC Med 2014;12:145
2. Kim PT, Testa G. Living donor liver transplantation in the USA. Hepatobiliary Surg Nutr 2016;5(2):133-40.
3. Roldan-Alzate A, Frydrychowicz A, Said A, Johnson KM, Francois CJ, Wieben O, et al. Impaired regulation of portal venous flow in response to a meal challenge as quantified by 4D flow MRI. J Magn Reson Imaging 2015;42(4):1009-17
4 Johnson KM, Lum DP, Turski PA, Block WF, Mistretta CA, Wieben O. Improved 3D phase contrast MRI with off-resonance corrected dual echo VIPR. Magn Reson Med 2008;60(6):1329-36.
5. Johnson KM, Wieben O, Samsonov AA. Phase-contrast velocimetry with simultaneous fat/water separation. Magn Reson Med 2010;63(6):1564-74.
6. Saidi RF, Jabbour N, Li Y, Shah SA, Bozorgzadeh A. Outcomes in partial liver transplantation: deceased donor split-liver vs. live donor liver transplantation. HPB (Oxford) 2011;13(11):797-801
Figures