0430
Diagnostic performance of LI-RADS major features, ancillary features, and categories on MRI for diagnosis of hepatocellular carcinoma1Department of Radiology, Centre hospitalier de l'Université de Montréal (CHUM), MONTREAL, QC, Canada, 2Department of Gastroenterology and Hepatology, Centre hospitalier de l'Université de Montréal (CHUM), MONTREAL, QC, Canada, 3Department of Surgery, Centre hospitalier de l'Université de Montréal (CHUM), MONTREAL, QC, Canada, 4Centre de recherche du Centre hospitalier de l'Université de Montréal (CRCHUM), MONTREAL, QC, Canada
Synopsis
Diagnosis of hepatocellular carcinoma (HCC) is largely based on imaging. MRI is ideally suited for the non-invasive diagnosis of HCC because it has numerous tissue contrast mechanisms and is the only modality that can assess all major and ancillary imaging features. We evaluated the diagnostic performance of MRI-determined LI-RADS major features, ancillary features, and categories for the diagnosis of HCC. Our results suggest that interpretation that includes ancillary features increases the sensitivity, while preserving a high specificity for definite HCC and a slightly lower specificity for probable HCC. Further, ancillary features in favor of benign entities have high specificity for benignity.
Introduction
Unlike other cancers, hepatocellular carcinoma (HCC) is often diagnosed based on imaging and pathology confirmation is not mandated by clinical guidelines.1-4 The Liver Imaging Reporting And Data System (LI-RADS) assigns categories reflecting the probability of HCC to liver observations based on the presence of major and ancillary imaging features in at-risk patients.5 While the accuracy of major features for HCC has been widely assessed,6-10 this has not been the case for ancillary features. Many studies have included ancillary features as part of sets of diagnostic criteria, however few have reported the diagnostic accuracy of ancillary features.9-11 MRI is ideally suited for the non-invasive diagnosis of HCC because it has numerous tissue contrast mechanisms and is the only modality that can assess all major and ancillary imaging features.
The aim was to evaluate the diagnostic accuracy of LI-RADS major features, ancillary features and LI-RADS categories for the diagnosis of HCC. We also assessed the impact of ancillary features on the final observation category.
Method
This retrospective, cross-sectional, single-site study was approved by our institutional review board. Patient consent was waived. Subjects were included in this study if: (1) they underwent a liver MRI performed with an extracellular contrast agent for clinical suspicion of HCC in a tertiary-care liver transplant center between October 2006 and March 2016, (2) the MRI was discussed in a multidisciplinary tumor board, and (3) there was at least one pathology-proven lesion per patient.
Of the 482 potentially eligible patients, 71 patients with 189 observations met the inclusion criteria. For each observation, the presence or absence of major and ancillary features was independently assessed by two abdominal radiologists blinded to clinical history and to the final diagnosis. LI-RADS v2014 categories were also assigned for each liver observation according to the major features alone and in combination with ancillary features. Disagreements were resolved by consensus. The composite reference standard was histopathology for 103 observations or follow-up imaging for 86 observations. The final diagnosis was HCC for 96 observations. Per-lesion diagnostic performance was estimated by sensitivity, specificity, accuracy, positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (PLR), and negative likelihood ratio (NLR). Estimates of diagnostic performance were calculated for major features, ancillary features, and LI-RADS categories.
Results
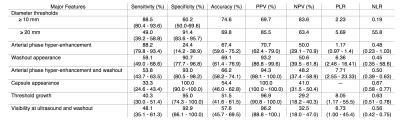
Table 1 summarizes the performance of major features for the diagnosis of HCC.
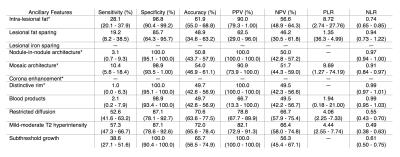
Table 2 summarizes the performance of ancillary features for the diagnosis of HCC.
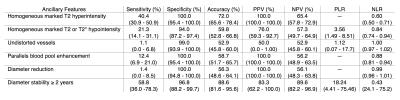
Table 3 summarizes the performance of ancillary features for the diagnosis of benignity.
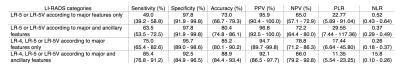
Table 4 summarizes the performance of LI-RADS categories according to major features alone or in combination with ancillary features for the diagnosis of HCC.
Ancillary features modified the final category in 24.9% (47/189) observations. The final category was upgraded in 14.8% (28/189) observations and downgraded in 10.1% (19/189) observations.
Discussion
1) Our results confirm that hallmark enhancement pattern (arterial phase hyper-enhancement and washout) and other major features (diameter ≥ 20 mm, capsule appearance, threshold growth, and visibility at ultrasound and washout) have high specificity (≥ 91.4%) and PPV (≥ 94.3%)
2) Ancillary features in favor of malignancy have low sensitivity (1.0% - 57.3%) but when present generally had high specificity (87.1% - 100.0%) for HCC. Nodule-in-nodule architecture, distinctive rim and subthreshold growth had excellent PPV (100.0%) for HCC.
3) Ancillary features in favor of benign entities have low sensitivity (1.1% - 58.8%) but when present had very high specificity (94.0% - 100.0%) for benignity. Homogeneous marked T2 hyperintensity, enhancement that parallels blood pool, and diameter reduction had excellent PPV (100.0%) for benignity.
4) For definite (LR-5 or LR-5V) HCC, interpretation that included ancillary features increased the sensitivity, but preserved a very high specificity for the diagnosis of HCC. For probable (LR-4) or definite (LR-5 and LR-5V) HCC, interpretation that included ancillary features also increased the sensitivity, but at the expense of lower specificity.
5) Our result are in agreement with those of previous studies by Rimola et al. and Kim et al. that have assessed the diagnostic performance of intra-lesional fat and mild-moderate T2 hyperintensity; however, we have provided estimates of diagnostic accuracy of ancillary features not previously assessed as stand-alone features.9,10
Conclusion
Our results indicate that an interpretation that includes ancillary features increases the sensitivity, while preserving a high specificity for definite HCC and a slightly lower specificity for probable HCC. Further, ancillary features in favor of benign entities have high specificity for benignity.Acknowledgements
An Tang is supported by a Career Award from the Fonds de recherche du Québec en Santé and Association des Radiologistes du Québec (FRQS-ARQ #26993) and a New Researcher Startup Grant from the Centre de Recherche du Centre Hospitalier de l'Université de Montréal (CRCHUM).References
1. Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020-2.
2. OPTN/UNOS policy 9: Allocation of Livers and Liver-Intestines. Available at: http://optn.transplant.hrsa.gov/ContentDocuments/OPTN_Policies.pdf - nameddest=Policy_09. 2015.
3. Mitchell DG, Bruix J, Sherman M, Sirlin CB. LI-RADS (Liver Imaging Reporting and Data System): summary, discussion, and consensus of the LI-RADS Management Working Group and future directions. Hepatology 2015;61:1056-65.
4. Omata M, Lesmana LA, Tateishi R, et al. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatology international 2010;4:439-74.
5. American College of Radiology. Liver Imaging Reporting and Data System version 2014. Most updated version available at: http://www.acr.org/Quality-Safety/Resources/LIRADS.
6. Burrel M, Llovet JM, Ayuso C, et al. MRI angiography is superior to helical CT for detection of HCC prior to liver transplantation: An explant correlation. Hepatology 2003;38:1034-42.
7. Sangiovanni A, Manini MA, Iavarone M, et al. The diagnostic and economic impact of contrast imaging techniques in the diagnosis of small hepatocellular carcinoma in cirrhosis. Gut 2010;59:638-44.
8. Forner A, Vilana R, Ayuso C, et al. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: Prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology 2008;47:97-104.
9. Rimola J, Forner A, Tremosini S, et al. Non-invasive diagnosis of hepatocellular carcinoma </= 2 cm in cirrhosis. Diagnostic accuracy assessing fat, capsule and signal intensity at dynamic MRI. J Hepatol 2012;56:1317-23.
10. Kim TK, Lee KH, Jang HJ, et al. Analysis of gadobenate dimeglumine-enhanced MR findings for characterizing small (1-2-cm) hepatic nodules in patients at high risk for hepatocellular carcinoma. Radiology 2011;259:730-8.
11. Nishie A, Yoshimitsu K, Asayama Y, et al. Radiologic detectability of minute portal venous invasion in hepatocellular carcinoma. AJR Am J Roentgenol 2008;190:81-7.
Figures