0421
DTI abnormalities in the midbrain in pediatric-onset multiple sclerosis.1Neurology, The Children's Hospital of Philadelphia, Philadelphia, PA, United States, 2Neurology, University of Pennsylvania, Philadelphia, PA, United States, 3Biology, University of Pennsylvania, Philadelphia, PA, United States, 4Psychology, York University, Toronto, ON, Canada, 5Hospital for Sick Children, Toronto, ON, Canada, 6Radiology, University of Pennsylvania, Philadelphia, PA, United States, 7Montreal Neurological Institute, McGill University, Montreal, QC, Canada, 8Neurology and Neurosurgery, Montreal Neurological Institute, McGill University, Montreal, QC, Canada
Synopsis
Pediatric-onset MS (POMS) is characterized by a high frequency of brainstem lesions early in the disease. It is unknown whether normal-appearing tissue in this region is involved at this early time point. Using diffusion tensor imaging (DTI) at 3T, we evaluated fractional anisotropy (FA) changes in midbrain substructures in POMS patients and age- and sex-matched healthy controls. Mean FA of midbrain was significantly reduced in the MS group, a difference that remained significant even after removing any focal midbrain lesions. Our results indicate a widespread disruption in the midbrain that exceeds lesional tissue disruption alone.
Introduction
Pediatric-onset MS (POMS) is characterized by frequent clinical relapses, high white matter lesion volumes relative to adult-onset disease, and by early and prominent lesional involvement in the brainstem. Diffusion tensor imaging (DTI) studies have demonstrated tissue disruption within lesions, and in normal-appearing brain tissue (NABT) in supratentorial regions. Little is known regarding the extent of nonlesional tissue disruption in the brainstem of these very early onset MS patients. Given the key role for brainstem pathways in motor, sensory and autonomic function, disruption of these pathways is likely to contribute to clinical symptoms and to the future risk of clinical disability. In adult-onset MS, brainstem pathology contributes substantively to clinical outcome. Given that pediatric MS patients rarely experience physical disability in the first 10 years of disease, we hypothesize that the NABT is indeed intact and able to compensate for the more focal lesions that occur early in the disease. We therefore performed brainstem DTI studies in POMS patients and compared the finding to healthy youth.Methods
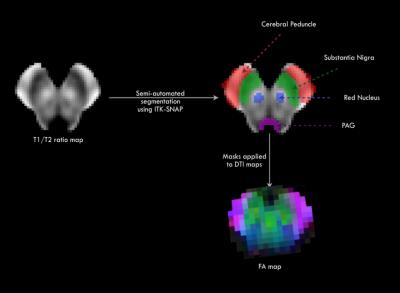
DTI studies were performed in 24 POMS (17 female, mean age at scan 18yrs±3SD, mean age at first MS attack 13 yrs±3SD, range 7 – 19 yrs) and 35 age- and sex-matched healthy controls (HC, 25 female, age at scan 18 yrs±3SD). The median expanded disability status scale (EDSS) score for the 24 patients was 1.5, (range 1-6). Only three of the patients required any form of ambulatory aid. T1-weighted MPRAGE (1mm3), T2 and FLAIR images (1x 1 x 3 mm3) and DTI images with 64 directions (voxel size = 2 x 2 x 3 mm3) were acquired on a single Siemens 3T Tim Trio MRI scanner with a 32-channel head coil. The MPRAGE image was processed and the midbrain was identified using freesurfer 5.3 toolkit1. For each subject, the FLAIR and T2 images were registered to the corresponding T1 MPRAGE image using boundary-based registration2. The T1/T2 ratio image provided the best contrast to visualize the various midbrain substructures. The DTI data was processed using FSL and denoised using a position orientation adaptive smoothing method3 to obtain mean diffusivity (MD), radial diffusivity (RD) and fractional anisotropy (FA) maps. The T1/T2 ratio, the midbrain segmentation and the DTI maps were normalized to an age appropriate pediatric asymmetric template4. A semi-automated method available in the ITK-SNAP toolkit5 was applied on the normalized T1/T2 map to segment the cerebral peduncles (CP), substantia nigra (SN), and red nucleus (RN). Mean FA, MD and RD values were computed from each of these regions for each subject. Owing to issues with DTI truncation in the lower pons in a few subjects, we elected to focus on the midbrain only.Results
10 of 24 POMS patients had lesions in the midbrain, which were subtracted prior to analysis. Mean FA of the entire midbrain NABT was reduced in MS subjects (FA= 0.3 (SD 0.02) vs HC FA: 0.32 (SD 0.02), p=0.009), and mean MD (1.06 X 10-3 (SD 0.04 X 10-3) vs HC 1.03 X 10-3 (SD 0.04 X 10-3), p=0.04) and mean RD (0.91 (SD 0.04) vs HC 0.88 (SD 0.04), p=0.02) were increased. Mean FA of the CP NABT was reduced in MS subjects ( 0.47 (SD 0.02) vs HC: 0.5 (SD 0.03), p=0.001), while the mean MD (0.97 X 10-3 (SD 0.05 X 10-3) vs HC 0.95 X 10-3 (SD 0.07 X 10-3), p=0.13) and RD (MS: 0.66 (SD 0.04) vs HC 0.63 (SD 0.05), p=0.015) did not differ from controls. Mean FA of the SN NABT was reduced (0.35 (SD 0.02) vs HC: 0.37 (SD 0.03), p=0.05), and mean RD was increased in MS subjects (0.63 (SD 0.03 X 10-3) vs HC 0.61 (SD 0.03 X 10-3), p=0.03). The mean MD did not differ by group (0.77 X 10-3 (SD 0.03 X 10-3) vs HC 0.76 X 10-3 (SD 0.02 X 10-3), p=0.13). DTI metrics in the RN NABT did not differ between MS patients and controls.Discussion
Despite very limited clinical disability in 21 of 24 POMS patients, DTI findings showed disruption of NABT in the midbrain. While the compensatory mechanisms involved in preservation of function remain to be defined, our findings are concerning in terms of the risk for future disability with increasing disease duration. It is possible that more eloquent tests of sensory function and more demanding motor performance evaluations might reveal correlative relationships between DTI and more subtle clinical impairment.Acknowledgements
Multiple Sclerosis Society of Canada
Scottish Rite Charitable Foundation
References
1. Iglesias JE, Van Leemput K, Bhatt P, Casillas C, Dutt S, Schuff N, Truran-Sacrey D, Boxer A, Fischl B, for the ADNI: "Bayesian segmentation of brainstem structures in MRI", NeuroImage, 113, June 2015, 184-195.
2. Greve DN, Fischl B. Accurate and robust brain image alignment using boundary-based registration. Neuroimage. 2009 Oct 15; 48(1):63-72.
3. Tabelow K, Mohammadi S, Weiskopf N, Polzehl J. POAS4SPM - a toolbox for SPM to denoise diffusion MRI data. NeuroInformatics, 2015, 13: 19-29.
4. Fonov V, Evans AC, Botteron K, Almli CR, McKinstry RC, Collins DL; Brain Development Cooperative Group. Unbiased average age-appropriate atlases for pediatric studies. Neuroimage. 2011 Jan 1;54(1):313-27.
5. Paul A. Yushkevich, Joseph Piven, Heather Cody Hazlett, Rachel Gimpel Smith, Sean Ho, James C. Gee, and Guido Gerig. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage 2006 Jul 1;31(3):1116-28.