0411
Comparison diffusion behavior of metabolites in brains of congenital portal systemic shunt and healthy mice in vivo at 14.1T1Laboratory of Functional and Metabolic Imaging (LIFMET), Ecole Polytechnique Fédérale de Lausanne, Lausanne, Switzerland, 2Center for Biomedical Imaging(CIBM), Ecole Polytechnique Fédérale de Lausanne, Lausanne, Switzerland, 3Department of Radiology, University of Geneva, Geneva, Switzerland
Synopsis
The aim of this study was to determine whether diffusion behavior of metabolites in the congenital portal systemic shunt(PSS) mouse brain is different from ones of the healthy controls in vivo, combining large diffusion weighting and 1H MRS methods. The diffusion behavior of most investigated metabolites except taurine was in excellent agreement with those in control mouse brain. The reduced diffusivities of taurine in PSS mice compared to control ones may be due of possible cellular redistribution of taurine in response to some osmotic perturbations in PSS mice in vivo, however, it needs to be further explored.
Purpose
Diffusion-weighted 1H MRS allows investigating the cellular compartmentalization of molecules in the living organs, e.g. brain1, and may shed insight on alterations of cellular restrictions faced by metabolites in different cerebral abnormalities and diseases. The neurochemical profile of congenital portal systemic shunt(PSS) mice displayed the abnormal neurochemical profile, consisting elevated glutamine(Gln) concentrations and reduced myo-inositol(Ins) concentrations2, both of which are highly located in astrocytes. Thus, we hypothesized that diffusion behavior of metabolites in PSS mouse brain might be altered compared to their control. Therefore, the aim of this study was to determine whether diffusion behavior of metabolites in the PSS mouse brain is different from ones of the healthy mouse in vivo, combining large diffusion weighting and 1H MRS methods.
Methods
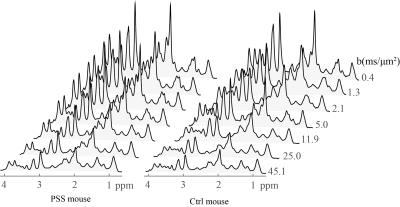
All experimental procedures involving animals were approved by the local veterinary authorities. All experiments were performed on a 14.1T/26cm horizontal magnet with a 12-cm gradient coil insert (400mT/m, 120μs) using a home-built proton quadrature transceiver. Six adult PSS and six age-matched healthy(Ctrl) C57BL/6J mice (male, 30-35g) have been prepared and anesthetized using 1-15.% isoflurane. 1H-MRS data were acquired using localized diffusion-weighed STEAM-based spectroscopic pulse sequence3 with optimum parameters(TE=16 ms and a mixing time of 113 ms1). Diffusion weighting was applied simultaneously along three orthogonal diffusion gradient directions (i.e. x, y and z) to cover the wide b range of 0 to 45 ms/ μm2. In vivo data was acquired with gradient duration of δ=6 ms, gradient separation of Δ=122 ms and gradient strength of 1, 13, 26, 52, 92, 143, 199 mT/m in a voxel of 91 μl (Fig 1) enabling sufficient sensitivity to correct the phase of single scan metabolite spectra at all b-values (Fig 2). Metabolite concentrations were quantified with LCModel. Data from the entire range of b-values in Fig 3 were fitted using following bi-exponential equation where ($$$D_{App}^{fast}$$$,$$$D_{App}^{slow}$$$) represent apparent diffusion coefficients of the fast and slow decaying signal components of each metabolite and $$$P_{App}^{slow}$$$ reflects the relative contribution of the slow component in the metabolite signal. S and S0 are signal intensity with and without diffusion gradients. $$\frac{S}{S_{0}}=P_{App}^{slow}.exp(-b.D_{App}^{slow})+(1-P_{App}^{slow}).exp(-b.D_{App}^{fast})$$
Results
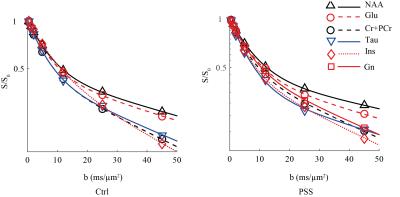
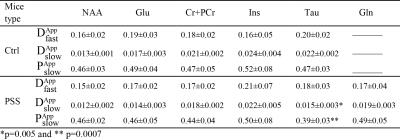
The remarkable sensitivity and spectral resolution of localized short-echo 1H MRS at 14T allowed a precise measurement of the diffusion properties of metabolites, e.g. N-actyle-aspartate(NAA), glutamate(Glu), total creatine(Cr+PCr), taurine(Tau) and Ins in the brain of PSS and Ctrl mouse in vivo at very high diffusion weighting(Fig 2). With such extensive diffusion weighting range, i.e. from 0 to 45 ms/μm2, a clear deviation from mono-exponential attenuation was observed for all investigated metabolites for both groups (Fig 3). $$$D_{App}^{fast}$$$ values of all investigated metabolites fell in the same range for both PSS and Ctrl groups(Table 1). Among all investigated metabolites, NAA showed the lowest $$$D_{App}^{slow}$$$. The elevated Gln levels in PSS mice allowed us to measure Gln diffusivity in the mouse brain in vivo. The $$$D_{App}^{fast}$$$ of Gln in PSS mouse brain was in the same range of other investigated metabolites. However, the $$$D_{App}^{slow}$$$ of Gln was higher than NAA(t-test, P<0.05), very similar to Ins and Cr+PCr(Table 1). The $$$D_{App}^{fast}$$$ and $$$D_{App}^{slow}$$$ values for most investigated metabolites(Table 1) were in excellent agreement with those in Ctrl mouse brain except that Tau showed slightly lowered $$$D_{App}^{slow}$$$ and $$$P_{App}^{slow}$$$ in PSS mice(t-test, p<0.05).
Discussion
A slight variation in $$$D_{App}^{slow}$$$ values between different metabolites suggested different intracellular distribution spaces for metabolites in the brain which was consistent with the proposed hypothesis about different localization of metabolites in the brain, such as NAA mostly in neurons4 and Ins mostly in astrocytes5. The diffusion behavior of Gln, similar to Ins but different from NAA, was in excellent agreement with the assumption made about considering a large pool of glutamine in astrocyte in 13C studies6, 7. The comparable diffusion properties of most investigated metabolites in the brain in vivo between PSS and Ctrl mice may indicate that unaltered barrier and cellular restriction dominate on the diffusion of metabolites in both group and therefore could support the hypothesis about the similarity of intracellular distribution space for these metabolites in PSS mice when compared to Ctrl mice. The reduced diffusivities of Tau in PSS mice compared to Ctrl ones may be due of possible cellular redistribution of Tau in response to some osmotic perturbations in PSS mice in vivo8,however, it needs to be further explored.
Conclusion
We conclude that diffusion behavior of a considerable number of metabolites in mouse brain can be assessed using large diffusion weighting and 1H-MRS methods at short echo time. Such method revealed the slightly different diffusivity of Tau in PSS mice when compared to controls.Acknowledgements
This study has been supported by Centre d’Imagerie BioMédicale (CIBM) of the UNIL, UNIGE, HUG, CHUV, EPFL, the Leenaards and Jeantet Foundations; SNF grant 131087.References
1. J. Pfeuffer, I. Tkác, and R. Gruetter, “Extracellular-intracellular distribution of glucose and lactate in the rat brain assessed noninvasively by diffusion-weighted 1H nuclear magnetic resonance spectroscopy in vivo,” J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab., vol. 20, no. 4, pp. 736–746, 2000.
2. C. Cudalbu et al., “The C57BL/6J Mouse Exhibits Sporadic Congenital Portosystemic Shunts,” PLoS ONE, vol. 8, no. 7, 2013.
3. N. Kunz, “Biophysical basis of the diffusion weighted magnetic resonance signal in the rat brain,” École polytechnique fédérale de Lausanne(EPFL), 2010.
4. Y. Assaf and Y. Cohen, “In vivo and in vitro bi-exponential diffusion of N-acetyl aspartate (NAA) in rat brain: a potential structural probe?,” NMR Biomed., vol. 11, no. 2, pp. 67–74, 1998.
5. A. Brand, C. Richter-Landsberg, and D. Leibfritz, “Multinuclear NMR studies on the energy metabolism of glial and neuronal cells,” Dev. Neurosci., vol. 15, no. 3–5, pp. 289–298, 1993.
6. M. Dehghani M., B. Lanz, J. M. N. Duarte, N. Kunz, and R. Gruetter, “Refined analysis of brain energy metabolism using in vivo dynamic enrichment of 13C multiplets,” ASN Neuro, vol. 8, no. 2, 2016.
7. R. Gruetter, “In vivo 13C NMR studies of compartmentalized cerebral carbohydrate metabolism,” Neurochem. Int., vol. 41, no. 2–3, pp. 143–154, 2002.
8. E. A. Nagelhus, A. Lehmann, and O. P. Ottersen, “Neuronal-glial exchange of taurine during hypo-osmotic stress: A combined immunocytochemical and biochemical analysis in rat cerebellar cortex,” Neuroscience, vol. 54, no. 3, pp. 615–631, 1993.
Figures