0401
HFE Mutations Alter White Matter Diffusion and Relaxation Parametrics in Alzheimer’s Disease1Neurosurgery, The Pennsylvania State University - College of Medicine, Hershey, PA, United States, 2Radiology, The Pennsylvania State University - College of Medicine, Hershey, PA, United States, 3Neurology, The Pennsylvania State University - College of Medicine, Hershey, PA, United States
Synopsis
This work demonstrates that HFE mutations in cognitively normal compared to wild-type subjects lead to differences in diffusion and relaxation parametrics, such that HFE mutation carrier parametrics converge towards AD subjects. Furthermore, HFE mutations appeared to be preservative against white matter integrity loss in AD patients. Iron-loading HFE mutations appear to preserve relaxation and diffusion neuroimaging biomarkers in AD patients, but adversely affect cognitively normal subjects.
Introduction
HFE polymorphisms have been implicated as a contributory factor in the development of neurodegenerative disorders, including Alzheimer’s disease (AD) (1). Mutations in this iron homeostatic gene may contribute to amyloidosis or the resulting inflammatory cycle, leading to neurodegeneration. Our previous work has shown reduced white matter relaxation and diffusion parametrics in cognitively normal HFE mutation carriers (2). It is hypothesized that HFE mutations in cognitively normal compared to wild-type subjects will lead to differences in neuroimaging data, such that HFE mutation carrier parametrics will converge towards AD subjects. Furthermore, it is hypothesized AD subjects with HFE mutations will have reduced white matter integrity compared to HFE-wild type (WT) AD carriersMethods
Cognitively normal (N=45, 27 HFE-H63D/+) and AD/MCI (N=26, 8 HFE-H63D/+) subjects were recruited for this study. All participants provided written informed consent according to IRB guidelines. Patients were imaged with a 3T Siemens Prisma MRI system, datasets included 3D-anatomical, multi-echo GE, multi-echo SE, and 70 direction DTI protocols. FA, MD, and MO diffusion tensor parametric maps were calculated from raw data using FSL’s diffusion toolbox and relaxation maps were computed using in house software (qMRI). SPM8 was used to register individual datasets, normalize these to the MNI template, generate VBM images using DARTEL, and conduct statistical analyses.Results
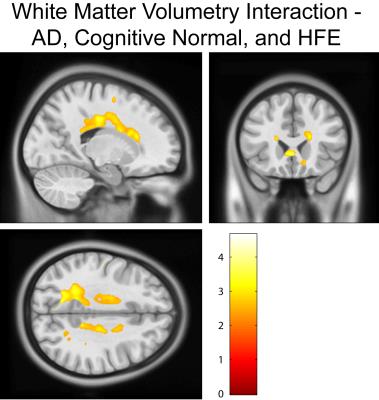
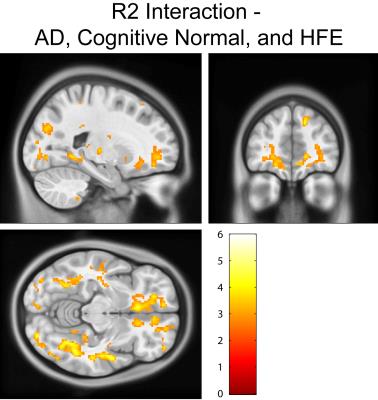
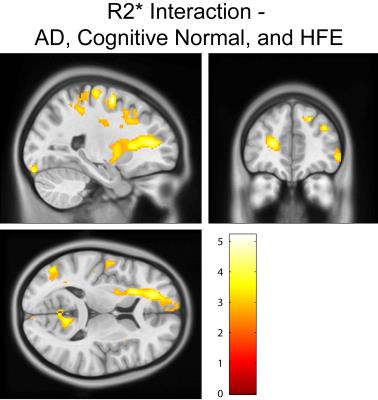
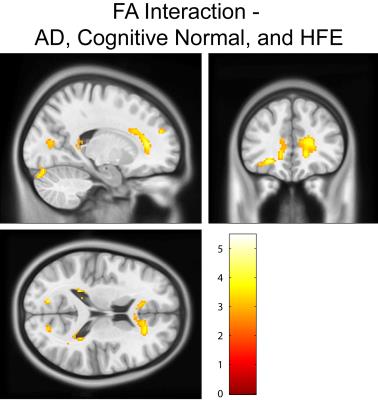
Cognitively normal subjects with HFE-H63D mutations exhibit MRI parametrics that converge towards AD metrics. Notably; in the prefrontal white matter, both relaxation (Figs. 1, 2, and 4) and diffusion metrics (Figs. 4 and 5) were shifted towards the AD group. The data demonstrate that AD HFE mutation carriers are not demonstrating a decrease in apparent white matter integrity compared to AD HFE-WT carriers. Of particular interest is the apparent reverse in the relationship, such that H63D-HFE mutations in AD patients appeared to be preservative for white matter parametric diffusion and relaxation biomarkers. The H63D-HFE AD group trends towards similarity with the cognitively normal subjects. The preservative effect was most significant in the same regions as observed in cognitively normal subjects, primarily the prefrontal WM and genu of the corpus callosum in both relaxation and DTI metrics. Statistical analysis of the interaction between AD status and genetics replicates these findings; HFE mutations have opposite trends in the cognitively normal and AD groups. For all comparisons, the majority of significant differences primarily appeared in major WM regions, rather than in cortical GM.Discussion
Although no difference was detected between the H63D-HFE and wild type AD patients in clinical cognitive scores, HFE mutations appeared to be somewhat preservative against neurological biomarkers of AD. Previous study of HFE knockout mice has demonstrated reduced expression of genes known to advance AD pathology, primarily APP, PSEN1, and Tau (3). HFE mutation induced dysfunction in iron homeostasis within nervous tissue may be related to AD trajectory (4). Mutant H63D-HFE expression appears related to AD-like white matter pathology in cognitively normal subjects. It is paradoxical that the same H63D-HFE mutation may be altering AD pathology towards preservation of white matter. Further study will look into the hypothesized cause of the WM observations as a result of oligodendrocyte myelination vulnerability to altered iron homeostasis.Conclusion
Iron-loading HFE mutations appear to preserve relaxation and diffusion neuroimaging biomarkers in AD patients, but adversely affect cognitively normal subjects. Alterations in neural iron homeostasis may be responsible for disrupting normal development and maturation, causing AD-like biomarkers, and disrupting standard AD progression; resulting in an apparent preservative effect rather than enhancing AD degeneration. Further research on the precise mechanism may lead to greater insight into the precise development of AD and mechanisms which may intervene in those processes.Acknowledgements
No acknowledgement found.References
1. Ali-Rahmani F, Schengrund CL, Connor JR. HFE gene variants, iron, and lipids: a novel connection in Alzheimer's disease. Front Pharmacol 2014;5:165.
2. Meadowcroft MD, Wang J, Purnell CJ, Peters DG, Eslinger PJ, Neely EB, Gill DJ, Vasavada M, Ali-Rahmani F, Yang QX, Connor JR. Reduced white matter MRI transverse relaxation rate in cognitively normal H63D-HFE human carriers and H67D-HFE mice. Brain Imaging Behav 2015.
3. Johnstone DM, Graham RM, Trinder D, Riveros C, Olynyk JK, Scott RJ, Moscato P, Milward EA. Changes in brain transcripts related to Alzheimer's disease in a model of HFE hemochromatosis are not consistent with increased Alzheimer's disease risk. J Alzheimers Dis 2012;30(4):791-803.
4. Wang ZX, Wan Y, Tan L, Liu J, Wang HF, Sun FR, Tan MS, Tan CC, Jiang T, Yu JT. Genetic Association of HLA Gene Variants with MRI Brain Structure in Alzheimer's Disease. Mol Neurobiol 2016.
Figures